

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50395220 CHEMBL2164664::US9150581, RTI-7527-(+/-)-154
SMILES: CS(=O)(=O)c1ccc(cc1)-c1cc(cnc1F)C1CC2CCC1N2
InChI Key: InChIKey=UYWOLUOTIQZQMZ-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Neuronal acetylcholine receptor subunit alpha-4 beta 2 (Rattus norvegicus (Rat)) | BDBM50395220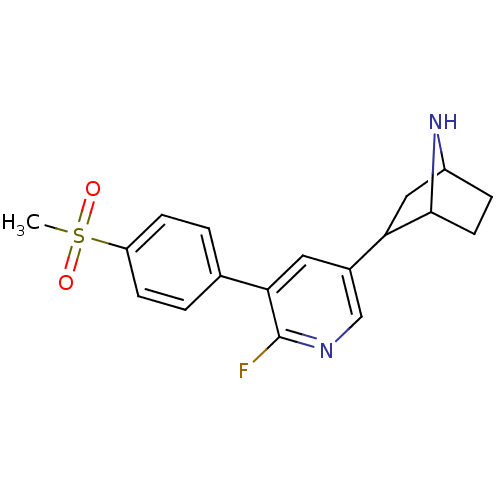 (CHEMBL2164664 | US9150581, RTI-7527-(+/-)-154) | PDB GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
RESEARCH TRIANGLE INSTITUTE; THE REGENTS OF THE UNIVERSITY OF MICHIGAN; VIRGINIA COMMONWEALTH UNIVERSITY US Patent | Assay Description The Ki values for the inhibition of [3H]epibatidine binding at the α4β2 nAChR in male rat cerebral cortex for compounds are listed in Table... | US Patent US9150581 (2015) BindingDB Entry DOI: 10.7270/Q2319TNR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4 beta 2 (Rattus norvegicus (Rat)) | BDBM50395220 (CHEMBL2164664 | US9150581, RTI-7527-(+/-)-154) | PDB GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 0.0820 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
RESEARCH TRIANGLE INSTITUTE; THE REGENTS OF THE UNIVERSITY OF MICHIGAN; VIRGINIA COMMONWEALTH UNIVERSITY US Patent | Assay Description The Ki values for the inhibition of [3H]epibatidine binding at the α4β2 nAChR in male rat cerebral cortex for compounds are listed in Table... | US Patent US9150581 (2015) BindingDB Entry DOI: 10.7270/Q2319TNR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4 beta 2 (Rattus norvegicus (Rat)) | BDBM50395220 (CHEMBL2164664 | US9150581, RTI-7527-(+/-)-154) | PDB GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
RESEARCH TRIANGLE INSTITUTE; THE REGENTS OF THE UNIVERSITY OF MICHIGAN; VIRGINIA COMMONWEALTH UNIVERSITY US Patent | Assay Description The Ki values for the inhibition of [3H]epibatidine binding at the α4β2 nAChR in male rat cerebral cortex for compounds are listed in Table... | US Patent US9150581 (2015) BindingDB Entry DOI: 10.7270/Q2319TNR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor (Rattus norvegicus (Rat)) | BDBM50395220 (CHEMBL2164664 | US9150581, RTI-7527-(+/-)-154) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
RESEARCH TRIANGLE INSTITUTE; THE REGENTS OF THE UNIVERSITY OF MICHIGAN; VIRGINIA COMMONWEALTH UNIVERSITY US Patent | Assay Description Compounds (10 mM) were also evaluated for inhibition of binding to a7 nAChR using [125I]iodoMLA as previously reported in Carroll et al. The binding ... | US Patent US9150581 (2015) BindingDB Entry DOI: 10.7270/Q2319TNR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor (Rattus norvegicus (Rat)) | BDBM50395220 (CHEMBL2164664 | US9150581, RTI-7527-(+/-)-154) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Displacement of [125I]iodoMLA from rat cerebral cortex alpha7 nAChR at 50 nM incubated for 2 hrs by microplate scintillation assay | J Med Chem 55: 6512-22 (2012) Article DOI: 10.1021/jm300575y BindingDB Entry DOI: 10.7270/Q2CV4JV0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor (Rattus norvegicus (Rat)) | BDBM50395220 (CHEMBL2164664 | US9150581, RTI-7527-(+/-)-154) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Antagonist activity at rat alpha7 nAChR expressed in Xenopus laevis oocytes assessed as inhibition of acetylcholine-induced response after 2 to 6 day... | J Med Chem 55: 6512-22 (2012) Article DOI: 10.1021/jm300575y BindingDB Entry DOI: 10.7270/Q2CV4JV0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||