 Found 16 hits for monomerid = 50396243
Found 16 hits for monomerid = 50396243 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
ALK tyrosine kinase receptor
(Homo sapiens (Human)) | BDBM50396243
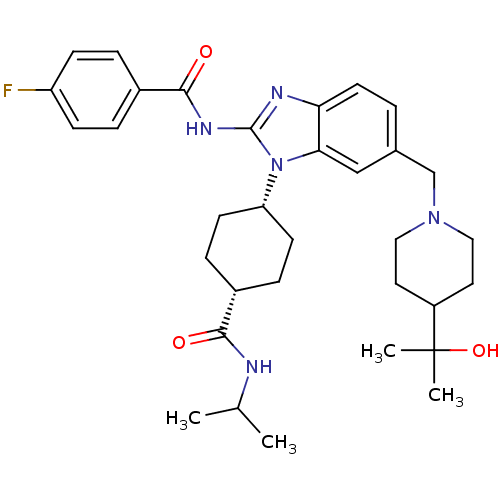
(CHEMBL2172308 | US10370379, Compound TSR-011)Show SMILES CC(C)NC(=O)[C@H]1CC[C@H](CC1)n1c(NC(=O)c2ccc(F)cc2)nc2ccc(CN3CCC(CC3)C(C)(C)O)cc12 |r,wU:9.12,6.5,(21.34,-25.87,;19.83,-25.56,;19.35,-24.09,;18.81,-26.7,;17.3,-26.39,;16.27,-27.53,;16.82,-24.92,;15.31,-24.6,;14.83,-23.15,;15.87,-22,;17.37,-22.31,;17.85,-23.77,;15.38,-20.54,;16.29,-19.28,;17.83,-19.27,;18.59,-17.94,;17.81,-16.61,;20.13,-17.93,;20.9,-19.25,;22.44,-19.25,;23.2,-17.91,;24.74,-17.9,;22.42,-16.57,;20.88,-16.59,;15.37,-18.03,;13.9,-18.52,;12.56,-17.76,;11.23,-18.53,;11.23,-20.07,;9.9,-20.84,;8.56,-20.07,;8.56,-18.53,;7.24,-17.76,;5.9,-18.52,;5.9,-20.06,;7.23,-20.84,;4.56,-17.74,;3.79,-16.41,;5.33,-16.4,;3.23,-18.51,;12.56,-20.84,;13.9,-20.07,)| Show InChI InChI=1S/C33H44FN5O3/c1-21(2)35-30(40)24-8-12-27(13-9-24)39-29-19-22(20-38-17-15-25(16-18-38)33(3,4)42)5-14-28(29)36-32(39)37-31(41)23-6-10-26(34)11-7-23/h5-7,10-11,14,19,21,24-25,27,42H,8-9,12-13,15-18,20H2,1-4H3,(H,35,40)(H,36,37,41)/t24-,27+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of ALK Tyr1604 phosphorylation by cell based assay |
J Med Chem 55: 6523-40 (2012)
Article DOI: 10.1021/jm3005866
BindingDB Entry DOI: 10.7270/Q2M046KT |
More data for this
Ligand-Target Pair | |
ALK tyrosine kinase receptor
(Homo sapiens (Human)) | BDBM50396243

(CHEMBL2172308 | US10370379, Compound TSR-011)Show SMILES CC(C)NC(=O)[C@H]1CC[C@H](CC1)n1c(NC(=O)c2ccc(F)cc2)nc2ccc(CN3CCC(CC3)C(C)(C)O)cc12 |r,wU:9.12,6.5,(21.34,-25.87,;19.83,-25.56,;19.35,-24.09,;18.81,-26.7,;17.3,-26.39,;16.27,-27.53,;16.82,-24.92,;15.31,-24.6,;14.83,-23.15,;15.87,-22,;17.37,-22.31,;17.85,-23.77,;15.38,-20.54,;16.29,-19.28,;17.83,-19.27,;18.59,-17.94,;17.81,-16.61,;20.13,-17.93,;20.9,-19.25,;22.44,-19.25,;23.2,-17.91,;24.74,-17.9,;22.42,-16.57,;20.88,-16.59,;15.37,-18.03,;13.9,-18.52,;12.56,-17.76,;11.23,-18.53,;11.23,-20.07,;9.9,-20.84,;8.56,-20.07,;8.56,-18.53,;7.24,-17.76,;5.9,-18.52,;5.9,-20.06,;7.23,-20.84,;4.56,-17.74,;3.79,-16.41,;5.33,-16.4,;3.23,-18.51,;12.56,-20.84,;13.9,-20.07,)| Show InChI InChI=1S/C33H44FN5O3/c1-21(2)35-30(40)24-8-12-27(13-9-24)39-29-19-22(20-38-17-15-25(16-18-38)33(3,4)42)5-14-28(29)36-32(39)37-31(41)23-6-10-26(34)11-7-23/h5-7,10-11,14,19,21,24-25,27,42H,8-9,12-13,15-18,20H2,1-4H3,(H,35,40)(H,36,37,41)/t24-,27+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University
Curated by ChEMBL
| Assay Description
Inhibition of ALK (unknown origin) |
J Med Chem 62: 10927-10954 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00446 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Mer
(Homo sapiens (Human)) | BDBM50396243

(CHEMBL2172308 | US10370379, Compound TSR-011)Show SMILES CC(C)NC(=O)[C@H]1CC[C@H](CC1)n1c(NC(=O)c2ccc(F)cc2)nc2ccc(CN3CCC(CC3)C(C)(C)O)cc12 |r,wU:9.12,6.5,(21.34,-25.87,;19.83,-25.56,;19.35,-24.09,;18.81,-26.7,;17.3,-26.39,;16.27,-27.53,;16.82,-24.92,;15.31,-24.6,;14.83,-23.15,;15.87,-22,;17.37,-22.31,;17.85,-23.77,;15.38,-20.54,;16.29,-19.28,;17.83,-19.27,;18.59,-17.94,;17.81,-16.61,;20.13,-17.93,;20.9,-19.25,;22.44,-19.25,;23.2,-17.91,;24.74,-17.9,;22.42,-16.57,;20.88,-16.59,;15.37,-18.03,;13.9,-18.52,;12.56,-17.76,;11.23,-18.53,;11.23,-20.07,;9.9,-20.84,;8.56,-20.07,;8.56,-18.53,;7.24,-17.76,;5.9,-18.52,;5.9,-20.06,;7.23,-20.84,;4.56,-17.74,;3.79,-16.41,;5.33,-16.4,;3.23,-18.51,;12.56,-20.84,;13.9,-20.07,)| Show InChI InChI=1S/C33H44FN5O3/c1-21(2)35-30(40)24-8-12-27(13-9-24)39-29-19-22(20-38-17-15-25(16-18-38)33(3,4)42)5-14-28(29)36-32(39)37-31(41)23-6-10-26(34)11-7-23/h5-7,10-11,14,19,21,24-25,27,42H,8-9,12-13,15-18,20H2,1-4H3,(H,35,40)(H,36,37,41)/t24-,27+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to human MER by Ambit titration assay |
J Med Chem 55: 6523-40 (2012)
Article DOI: 10.1021/jm3005866
BindingDB Entry DOI: 10.7270/Q2M046KT |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 2
(Homo sapiens (Human)) | BDBM50396243

(CHEMBL2172308 | US10370379, Compound TSR-011)Show SMILES CC(C)NC(=O)[C@H]1CC[C@H](CC1)n1c(NC(=O)c2ccc(F)cc2)nc2ccc(CN3CCC(CC3)C(C)(C)O)cc12 |r,wU:9.12,6.5,(21.34,-25.87,;19.83,-25.56,;19.35,-24.09,;18.81,-26.7,;17.3,-26.39,;16.27,-27.53,;16.82,-24.92,;15.31,-24.6,;14.83,-23.15,;15.87,-22,;17.37,-22.31,;17.85,-23.77,;15.38,-20.54,;16.29,-19.28,;17.83,-19.27,;18.59,-17.94,;17.81,-16.61,;20.13,-17.93,;20.9,-19.25,;22.44,-19.25,;23.2,-17.91,;24.74,-17.9,;22.42,-16.57,;20.88,-16.59,;15.37,-18.03,;13.9,-18.52,;12.56,-17.76,;11.23,-18.53,;11.23,-20.07,;9.9,-20.84,;8.56,-20.07,;8.56,-18.53,;7.24,-17.76,;5.9,-18.52,;5.9,-20.06,;7.23,-20.84,;4.56,-17.74,;3.79,-16.41,;5.33,-16.4,;3.23,-18.51,;12.56,-20.84,;13.9,-20.07,)| Show InChI InChI=1S/C33H44FN5O3/c1-21(2)35-30(40)24-8-12-27(13-9-24)39-29-19-22(20-38-17-15-25(16-18-38)33(3,4)42)5-14-28(29)36-32(39)37-31(41)23-6-10-26(34)11-7-23/h5-7,10-11,14,19,21,24-25,27,42H,8-9,12-13,15-18,20H2,1-4H3,(H,35,40)(H,36,37,41)/t24-,27+ | MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to human MAP4K2 by Ambit titration assay |
J Med Chem 55: 6523-40 (2012)
Article DOI: 10.1021/jm3005866
BindingDB Entry DOI: 10.7270/Q2M046KT |
More data for this
Ligand-Target Pair | |
Leukocyte tyrosine kinase receptor
(Homo sapiens (Human)) | BDBM50396243

(CHEMBL2172308 | US10370379, Compound TSR-011)Show SMILES CC(C)NC(=O)[C@H]1CC[C@H](CC1)n1c(NC(=O)c2ccc(F)cc2)nc2ccc(CN3CCC(CC3)C(C)(C)O)cc12 |r,wU:9.12,6.5,(21.34,-25.87,;19.83,-25.56,;19.35,-24.09,;18.81,-26.7,;17.3,-26.39,;16.27,-27.53,;16.82,-24.92,;15.31,-24.6,;14.83,-23.15,;15.87,-22,;17.37,-22.31,;17.85,-23.77,;15.38,-20.54,;16.29,-19.28,;17.83,-19.27,;18.59,-17.94,;17.81,-16.61,;20.13,-17.93,;20.9,-19.25,;22.44,-19.25,;23.2,-17.91,;24.74,-17.9,;22.42,-16.57,;20.88,-16.59,;15.37,-18.03,;13.9,-18.52,;12.56,-17.76,;11.23,-18.53,;11.23,-20.07,;9.9,-20.84,;8.56,-20.07,;8.56,-18.53,;7.24,-17.76,;5.9,-18.52,;5.9,-20.06,;7.23,-20.84,;4.56,-17.74,;3.79,-16.41,;5.33,-16.4,;3.23,-18.51,;12.56,-20.84,;13.9,-20.07,)| Show InChI InChI=1S/C33H44FN5O3/c1-21(2)35-30(40)24-8-12-27(13-9-24)39-29-19-22(20-38-17-15-25(16-18-38)33(3,4)42)5-14-28(29)36-32(39)37-31(41)23-6-10-26(34)11-7-23/h5-7,10-11,14,19,21,24-25,27,42H,8-9,12-13,15-18,20H2,1-4H3,(H,35,40)(H,36,37,41)/t24-,27+ | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to human LTK by Ambit titration assay |
J Med Chem 55: 6523-40 (2012)
Article DOI: 10.1021/jm3005866
BindingDB Entry DOI: 10.7270/Q2M046KT |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50396243

(CHEMBL2172308 | US10370379, Compound TSR-011)Show SMILES CC(C)NC(=O)[C@H]1CC[C@H](CC1)n1c(NC(=O)c2ccc(F)cc2)nc2ccc(CN3CCC(CC3)C(C)(C)O)cc12 |r,wU:9.12,6.5,(21.34,-25.87,;19.83,-25.56,;19.35,-24.09,;18.81,-26.7,;17.3,-26.39,;16.27,-27.53,;16.82,-24.92,;15.31,-24.6,;14.83,-23.15,;15.87,-22,;17.37,-22.31,;17.85,-23.77,;15.38,-20.54,;16.29,-19.28,;17.83,-19.27,;18.59,-17.94,;17.81,-16.61,;20.13,-17.93,;20.9,-19.25,;22.44,-19.25,;23.2,-17.91,;24.74,-17.9,;22.42,-16.57,;20.88,-16.59,;15.37,-18.03,;13.9,-18.52,;12.56,-17.76,;11.23,-18.53,;11.23,-20.07,;9.9,-20.84,;8.56,-20.07,;8.56,-18.53,;7.24,-17.76,;5.9,-18.52,;5.9,-20.06,;7.23,-20.84,;4.56,-17.74,;3.79,-16.41,;5.33,-16.4,;3.23,-18.51,;12.56,-20.84,;13.9,-20.07,)| Show InChI InChI=1S/C33H44FN5O3/c1-21(2)35-30(40)24-8-12-27(13-9-24)39-29-19-22(20-38-17-15-25(16-18-38)33(3,4)42)5-14-28(29)36-32(39)37-31(41)23-6-10-26(34)11-7-23/h5-7,10-11,14,19,21,24-25,27,42H,8-9,12-13,15-18,20H2,1-4H3,(H,35,40)(H,36,37,41)/t24-,27+ | PDB
UniProtKB/SwissProt
UniProtKB/TrEMBL
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to human LCK by Ambit titration assay |
J Med Chem 55: 6523-40 (2012)
Article DOI: 10.1021/jm3005866
BindingDB Entry DOI: 10.7270/Q2M046KT |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 1
(Homo sapiens (Human)) | BDBM50396243

(CHEMBL2172308 | US10370379, Compound TSR-011)Show SMILES CC(C)NC(=O)[C@H]1CC[C@H](CC1)n1c(NC(=O)c2ccc(F)cc2)nc2ccc(CN3CCC(CC3)C(C)(C)O)cc12 |r,wU:9.12,6.5,(21.34,-25.87,;19.83,-25.56,;19.35,-24.09,;18.81,-26.7,;17.3,-26.39,;16.27,-27.53,;16.82,-24.92,;15.31,-24.6,;14.83,-23.15,;15.87,-22,;17.37,-22.31,;17.85,-23.77,;15.38,-20.54,;16.29,-19.28,;17.83,-19.27,;18.59,-17.94,;17.81,-16.61,;20.13,-17.93,;20.9,-19.25,;22.44,-19.25,;23.2,-17.91,;24.74,-17.9,;22.42,-16.57,;20.88,-16.59,;15.37,-18.03,;13.9,-18.52,;12.56,-17.76,;11.23,-18.53,;11.23,-20.07,;9.9,-20.84,;8.56,-20.07,;8.56,-18.53,;7.24,-17.76,;5.9,-18.52,;5.9,-20.06,;7.23,-20.84,;4.56,-17.74,;3.79,-16.41,;5.33,-16.4,;3.23,-18.51,;12.56,-20.84,;13.9,-20.07,)| Show InChI InChI=1S/C33H44FN5O3/c1-21(2)35-30(40)24-8-12-27(13-9-24)39-29-19-22(20-38-17-15-25(16-18-38)33(3,4)42)5-14-28(29)36-32(39)37-31(41)23-6-10-26(34)11-7-23/h5-7,10-11,14,19,21,24-25,27,42H,8-9,12-13,15-18,20H2,1-4H3,(H,35,40)(H,36,37,41)/t24-,27+ | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 5.70 | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to human IRAK1 by Ambit titration assay |
J Med Chem 55: 6523-40 (2012)
Article DOI: 10.1021/jm3005866
BindingDB Entry DOI: 10.7270/Q2M046KT |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50396243

(CHEMBL2172308 | US10370379, Compound TSR-011)Show SMILES CC(C)NC(=O)[C@H]1CC[C@H](CC1)n1c(NC(=O)c2ccc(F)cc2)nc2ccc(CN3CCC(CC3)C(C)(C)O)cc12 |r,wU:9.12,6.5,(21.34,-25.87,;19.83,-25.56,;19.35,-24.09,;18.81,-26.7,;17.3,-26.39,;16.27,-27.53,;16.82,-24.92,;15.31,-24.6,;14.83,-23.15,;15.87,-22,;17.37,-22.31,;17.85,-23.77,;15.38,-20.54,;16.29,-19.28,;17.83,-19.27,;18.59,-17.94,;17.81,-16.61,;20.13,-17.93,;20.9,-19.25,;22.44,-19.25,;23.2,-17.91,;24.74,-17.9,;22.42,-16.57,;20.88,-16.59,;15.37,-18.03,;13.9,-18.52,;12.56,-17.76,;11.23,-18.53,;11.23,-20.07,;9.9,-20.84,;8.56,-20.07,;8.56,-18.53,;7.24,-17.76,;5.9,-18.52,;5.9,-20.06,;7.23,-20.84,;4.56,-17.74,;3.79,-16.41,;5.33,-16.4,;3.23,-18.51,;12.56,-20.84,;13.9,-20.07,)| Show InChI InChI=1S/C33H44FN5O3/c1-21(2)35-30(40)24-8-12-27(13-9-24)39-29-19-22(20-38-17-15-25(16-18-38)33(3,4)42)5-14-28(29)36-32(39)37-31(41)23-6-10-26(34)11-7-23/h5-7,10-11,14,19,21,24-25,27,42H,8-9,12-13,15-18,20H2,1-4H3,(H,35,40)(H,36,37,41)/t24-,27+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to human FLT3 by Ambit titration assay |
J Med Chem 55: 6523-40 (2012)
Article DOI: 10.1021/jm3005866
BindingDB Entry DOI: 10.7270/Q2M046KT |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase receptor UFO
(Homo sapiens (Human)) | BDBM50396243

(CHEMBL2172308 | US10370379, Compound TSR-011)Show SMILES CC(C)NC(=O)[C@H]1CC[C@H](CC1)n1c(NC(=O)c2ccc(F)cc2)nc2ccc(CN3CCC(CC3)C(C)(C)O)cc12 |r,wU:9.12,6.5,(21.34,-25.87,;19.83,-25.56,;19.35,-24.09,;18.81,-26.7,;17.3,-26.39,;16.27,-27.53,;16.82,-24.92,;15.31,-24.6,;14.83,-23.15,;15.87,-22,;17.37,-22.31,;17.85,-23.77,;15.38,-20.54,;16.29,-19.28,;17.83,-19.27,;18.59,-17.94,;17.81,-16.61,;20.13,-17.93,;20.9,-19.25,;22.44,-19.25,;23.2,-17.91,;24.74,-17.9,;22.42,-16.57,;20.88,-16.59,;15.37,-18.03,;13.9,-18.52,;12.56,-17.76,;11.23,-18.53,;11.23,-20.07,;9.9,-20.84,;8.56,-20.07,;8.56,-18.53,;7.24,-17.76,;5.9,-18.52,;5.9,-20.06,;7.23,-20.84,;4.56,-17.74,;3.79,-16.41,;5.33,-16.4,;3.23,-18.51,;12.56,-20.84,;13.9,-20.07,)| Show InChI InChI=1S/C33H44FN5O3/c1-21(2)35-30(40)24-8-12-27(13-9-24)39-29-19-22(20-38-17-15-25(16-18-38)33(3,4)42)5-14-28(29)36-32(39)37-31(41)23-6-10-26(34)11-7-23/h5-7,10-11,14,19,21,24-25,27,42H,8-9,12-13,15-18,20H2,1-4H3,(H,35,40)(H,36,37,41)/t24-,27+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to human AXL by Ambit titration assay |
J Med Chem 55: 6523-40 (2012)
Article DOI: 10.1021/jm3005866
BindingDB Entry DOI: 10.7270/Q2M046KT |
More data for this
Ligand-Target Pair | |
Insulin receptor
(Homo sapiens (Human)) | BDBM50396243

(CHEMBL2172308 | US10370379, Compound TSR-011)Show SMILES CC(C)NC(=O)[C@H]1CC[C@H](CC1)n1c(NC(=O)c2ccc(F)cc2)nc2ccc(CN3CCC(CC3)C(C)(C)O)cc12 |r,wU:9.12,6.5,(21.34,-25.87,;19.83,-25.56,;19.35,-24.09,;18.81,-26.7,;17.3,-26.39,;16.27,-27.53,;16.82,-24.92,;15.31,-24.6,;14.83,-23.15,;15.87,-22,;17.37,-22.31,;17.85,-23.77,;15.38,-20.54,;16.29,-19.28,;17.83,-19.27,;18.59,-17.94,;17.81,-16.61,;20.13,-17.93,;20.9,-19.25,;22.44,-19.25,;23.2,-17.91,;24.74,-17.9,;22.42,-16.57,;20.88,-16.59,;15.37,-18.03,;13.9,-18.52,;12.56,-17.76,;11.23,-18.53,;11.23,-20.07,;9.9,-20.84,;8.56,-20.07,;8.56,-18.53,;7.24,-17.76,;5.9,-18.52,;5.9,-20.06,;7.23,-20.84,;4.56,-17.74,;3.79,-16.41,;5.33,-16.4,;3.23,-18.51,;12.56,-20.84,;13.9,-20.07,)| Show InChI InChI=1S/C33H44FN5O3/c1-21(2)35-30(40)24-8-12-27(13-9-24)39-29-19-22(20-38-17-15-25(16-18-38)33(3,4)42)5-14-28(29)36-32(39)37-31(41)23-6-10-26(34)11-7-23/h5-7,10-11,14,19,21,24-25,27,42H,8-9,12-13,15-18,20H2,1-4H3,(H,35,40)(H,36,37,41)/t24-,27+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 231 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of INSR expressed in CHO cells assessed as inhibition of receptor phosphorylation pre-incubated before insulin stimulation by Meso-Scale D... |
J Med Chem 55: 6523-40 (2012)
Article DOI: 10.1021/jm3005866
BindingDB Entry DOI: 10.7270/Q2M046KT |
More data for this
Ligand-Target Pair | |
ALK tyrosine kinase receptor
(Homo sapiens (Human)) | BDBM50396243

(CHEMBL2172308 | US10370379, Compound TSR-011)Show SMILES CC(C)NC(=O)[C@H]1CC[C@H](CC1)n1c(NC(=O)c2ccc(F)cc2)nc2ccc(CN3CCC(CC3)C(C)(C)O)cc12 |r,wU:9.12,6.5,(21.34,-25.87,;19.83,-25.56,;19.35,-24.09,;18.81,-26.7,;17.3,-26.39,;16.27,-27.53,;16.82,-24.92,;15.31,-24.6,;14.83,-23.15,;15.87,-22,;17.37,-22.31,;17.85,-23.77,;15.38,-20.54,;16.29,-19.28,;17.83,-19.27,;18.59,-17.94,;17.81,-16.61,;20.13,-17.93,;20.9,-19.25,;22.44,-19.25,;23.2,-17.91,;24.74,-17.9,;22.42,-16.57,;20.88,-16.59,;15.37,-18.03,;13.9,-18.52,;12.56,-17.76,;11.23,-18.53,;11.23,-20.07,;9.9,-20.84,;8.56,-20.07,;8.56,-18.53,;7.24,-17.76,;5.9,-18.52,;5.9,-20.06,;7.23,-20.84,;4.56,-17.74,;3.79,-16.41,;5.33,-16.4,;3.23,-18.51,;12.56,-20.84,;13.9,-20.07,)| Show InChI InChI=1S/C33H44FN5O3/c1-21(2)35-30(40)24-8-12-27(13-9-24)39-29-19-22(20-38-17-15-25(16-18-38)33(3,4)42)5-14-28(29)36-32(39)37-31(41)23-6-10-26(34)11-7-23/h5-7,10-11,14,19,21,24-25,27,42H,8-9,12-13,15-18,20H2,1-4H3,(H,35,40)(H,36,37,41)/t24-,27+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of ALK enzyme |
J Med Chem 55: 6523-40 (2012)
Article DOI: 10.1021/jm3005866
BindingDB Entry DOI: 10.7270/Q2M046KT |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM50396243

(CHEMBL2172308 | US10370379, Compound TSR-011)Show SMILES CC(C)NC(=O)[C@H]1CC[C@H](CC1)n1c(NC(=O)c2ccc(F)cc2)nc2ccc(CN3CCC(CC3)C(C)(C)O)cc12 |r,wU:9.12,6.5,(21.34,-25.87,;19.83,-25.56,;19.35,-24.09,;18.81,-26.7,;17.3,-26.39,;16.27,-27.53,;16.82,-24.92,;15.31,-24.6,;14.83,-23.15,;15.87,-22,;17.37,-22.31,;17.85,-23.77,;15.38,-20.54,;16.29,-19.28,;17.83,-19.27,;18.59,-17.94,;17.81,-16.61,;20.13,-17.93,;20.9,-19.25,;22.44,-19.25,;23.2,-17.91,;24.74,-17.9,;22.42,-16.57,;20.88,-16.59,;15.37,-18.03,;13.9,-18.52,;12.56,-17.76,;11.23,-18.53,;11.23,-20.07,;9.9,-20.84,;8.56,-20.07,;8.56,-18.53,;7.24,-17.76,;5.9,-18.52,;5.9,-20.06,;7.23,-20.84,;4.56,-17.74,;3.79,-16.41,;5.33,-16.4,;3.23,-18.51,;12.56,-20.84,;13.9,-20.07,)| Show InChI InChI=1S/C33H44FN5O3/c1-21(2)35-30(40)24-8-12-27(13-9-24)39-29-19-22(20-38-17-15-25(16-18-38)33(3,4)42)5-14-28(29)36-32(39)37-31(41)23-6-10-26(34)11-7-23/h5-7,10-11,14,19,21,24-25,27,42H,8-9,12-13,15-18,20H2,1-4H3,(H,35,40)(H,36,37,41)/t24-,27+ | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
BLUEPRINT MEDICINES CORPORATION
US Patent
| Assay Description
In each well of a 384-well plate, 1 nM-1.5 nM of wild type NTRK1 enzyme (BPS Bioscience; 40280) was incubated in a total of 12.5 μL of buffer (1... |
US Patent US10370379 (2019)
BindingDB Entry DOI: 10.7270/Q2TM7DG5 |
More data for this
Ligand-Target Pair | |
NT-3 growth factor receptor
(Homo sapiens (Human)) | BDBM50396243

(CHEMBL2172308 | US10370379, Compound TSR-011)Show SMILES CC(C)NC(=O)[C@H]1CC[C@H](CC1)n1c(NC(=O)c2ccc(F)cc2)nc2ccc(CN3CCC(CC3)C(C)(C)O)cc12 |r,wU:9.12,6.5,(21.34,-25.87,;19.83,-25.56,;19.35,-24.09,;18.81,-26.7,;17.3,-26.39,;16.27,-27.53,;16.82,-24.92,;15.31,-24.6,;14.83,-23.15,;15.87,-22,;17.37,-22.31,;17.85,-23.77,;15.38,-20.54,;16.29,-19.28,;17.83,-19.27,;18.59,-17.94,;17.81,-16.61,;20.13,-17.93,;20.9,-19.25,;22.44,-19.25,;23.2,-17.91,;24.74,-17.9,;22.42,-16.57,;20.88,-16.59,;15.37,-18.03,;13.9,-18.52,;12.56,-17.76,;11.23,-18.53,;11.23,-20.07,;9.9,-20.84,;8.56,-20.07,;8.56,-18.53,;7.24,-17.76,;5.9,-18.52,;5.9,-20.06,;7.23,-20.84,;4.56,-17.74,;3.79,-16.41,;5.33,-16.4,;3.23,-18.51,;12.56,-20.84,;13.9,-20.07,)| Show InChI InChI=1S/C33H44FN5O3/c1-21(2)35-30(40)24-8-12-27(13-9-24)39-29-19-22(20-38-17-15-25(16-18-38)33(3,4)42)5-14-28(29)36-32(39)37-31(41)23-6-10-26(34)11-7-23/h5-7,10-11,14,19,21,24-25,27,42H,8-9,12-13,15-18,20H2,1-4H3,(H,35,40)(H,36,37,41)/t24-,27+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arkansas for Medical Sciences
Curated by ChEMBL
| Assay Description
Inhibition of TRKC (unknown origin) |
J Med Chem 62: 1731-1760 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01092 |
More data for this
Ligand-Target Pair | |
BDNF/NT-3 growth factors receptor
(Homo sapiens (Human)) | BDBM50396243

(CHEMBL2172308 | US10370379, Compound TSR-011)Show SMILES CC(C)NC(=O)[C@H]1CC[C@H](CC1)n1c(NC(=O)c2ccc(F)cc2)nc2ccc(CN3CCC(CC3)C(C)(C)O)cc12 |r,wU:9.12,6.5,(21.34,-25.87,;19.83,-25.56,;19.35,-24.09,;18.81,-26.7,;17.3,-26.39,;16.27,-27.53,;16.82,-24.92,;15.31,-24.6,;14.83,-23.15,;15.87,-22,;17.37,-22.31,;17.85,-23.77,;15.38,-20.54,;16.29,-19.28,;17.83,-19.27,;18.59,-17.94,;17.81,-16.61,;20.13,-17.93,;20.9,-19.25,;22.44,-19.25,;23.2,-17.91,;24.74,-17.9,;22.42,-16.57,;20.88,-16.59,;15.37,-18.03,;13.9,-18.52,;12.56,-17.76,;11.23,-18.53,;11.23,-20.07,;9.9,-20.84,;8.56,-20.07,;8.56,-18.53,;7.24,-17.76,;5.9,-18.52,;5.9,-20.06,;7.23,-20.84,;4.56,-17.74,;3.79,-16.41,;5.33,-16.4,;3.23,-18.51,;12.56,-20.84,;13.9,-20.07,)| Show InChI InChI=1S/C33H44FN5O3/c1-21(2)35-30(40)24-8-12-27(13-9-24)39-29-19-22(20-38-17-15-25(16-18-38)33(3,4)42)5-14-28(29)36-32(39)37-31(41)23-6-10-26(34)11-7-23/h5-7,10-11,14,19,21,24-25,27,42H,8-9,12-13,15-18,20H2,1-4H3,(H,35,40)(H,36,37,41)/t24-,27+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arkansas for Medical Sciences
Curated by ChEMBL
| Assay Description
Inhibition of TRKB (unknown origin) |
J Med Chem 62: 1731-1760 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01092 |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM50396243

(CHEMBL2172308 | US10370379, Compound TSR-011)Show SMILES CC(C)NC(=O)[C@H]1CC[C@H](CC1)n1c(NC(=O)c2ccc(F)cc2)nc2ccc(CN3CCC(CC3)C(C)(C)O)cc12 |r,wU:9.12,6.5,(21.34,-25.87,;19.83,-25.56,;19.35,-24.09,;18.81,-26.7,;17.3,-26.39,;16.27,-27.53,;16.82,-24.92,;15.31,-24.6,;14.83,-23.15,;15.87,-22,;17.37,-22.31,;17.85,-23.77,;15.38,-20.54,;16.29,-19.28,;17.83,-19.27,;18.59,-17.94,;17.81,-16.61,;20.13,-17.93,;20.9,-19.25,;22.44,-19.25,;23.2,-17.91,;24.74,-17.9,;22.42,-16.57,;20.88,-16.59,;15.37,-18.03,;13.9,-18.52,;12.56,-17.76,;11.23,-18.53,;11.23,-20.07,;9.9,-20.84,;8.56,-20.07,;8.56,-18.53,;7.24,-17.76,;5.9,-18.52,;5.9,-20.06,;7.23,-20.84,;4.56,-17.74,;3.79,-16.41,;5.33,-16.4,;3.23,-18.51,;12.56,-20.84,;13.9,-20.07,)| Show InChI InChI=1S/C33H44FN5O3/c1-21(2)35-30(40)24-8-12-27(13-9-24)39-29-19-22(20-38-17-15-25(16-18-38)33(3,4)42)5-14-28(29)36-32(39)37-31(41)23-6-10-26(34)11-7-23/h5-7,10-11,14,19,21,24-25,27,42H,8-9,12-13,15-18,20H2,1-4H3,(H,35,40)(H,36,37,41)/t24-,27+ | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arkansas for Medical Sciences
Curated by ChEMBL
| Assay Description
Inhibition of TRKA (unknown origin) |
J Med Chem 62: 1731-1760 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01092 |
More data for this
Ligand-Target Pair | |
ALK tyrosine kinase receptor
(Homo sapiens (Human)) | BDBM50396243

(CHEMBL2172308 | US10370379, Compound TSR-011)Show SMILES CC(C)NC(=O)[C@H]1CC[C@H](CC1)n1c(NC(=O)c2ccc(F)cc2)nc2ccc(CN3CCC(CC3)C(C)(C)O)cc12 |r,wU:9.12,6.5,(21.34,-25.87,;19.83,-25.56,;19.35,-24.09,;18.81,-26.7,;17.3,-26.39,;16.27,-27.53,;16.82,-24.92,;15.31,-24.6,;14.83,-23.15,;15.87,-22,;17.37,-22.31,;17.85,-23.77,;15.38,-20.54,;16.29,-19.28,;17.83,-19.27,;18.59,-17.94,;17.81,-16.61,;20.13,-17.93,;20.9,-19.25,;22.44,-19.25,;23.2,-17.91,;24.74,-17.9,;22.42,-16.57,;20.88,-16.59,;15.37,-18.03,;13.9,-18.52,;12.56,-17.76,;11.23,-18.53,;11.23,-20.07,;9.9,-20.84,;8.56,-20.07,;8.56,-18.53,;7.24,-17.76,;5.9,-18.52,;5.9,-20.06,;7.23,-20.84,;4.56,-17.74,;3.79,-16.41,;5.33,-16.4,;3.23,-18.51,;12.56,-20.84,;13.9,-20.07,)| Show InChI InChI=1S/C33H44FN5O3/c1-21(2)35-30(40)24-8-12-27(13-9-24)39-29-19-22(20-38-17-15-25(16-18-38)33(3,4)42)5-14-28(29)36-32(39)37-31(41)23-6-10-26(34)11-7-23/h5-7,10-11,14,19,21,24-25,27,42H,8-9,12-13,15-18,20H2,1-4H3,(H,35,40)(H,36,37,41)/t24-,27+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 0.360 | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to human ALK by Ambit titration assay |
J Med Chem 55: 6523-40 (2012)
Article DOI: 10.1021/jm3005866
BindingDB Entry DOI: 10.7270/Q2M046KT |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data