

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50396443 CHEMBL2170632
SMILES: CS(=O)(=O)c1ccc(cc1)C(=O)N1CCC(CC1)c1ccc(cc1C(F)(F)F)C(=O)NC(N)=N
InChI Key: InChIKey=TZUIJGLTRUKCJZ-UHFFFAOYSA-N
Data: 6 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sodium/hydrogen exchanger 1 (Homo sapiens (Human)) | BDBM50396443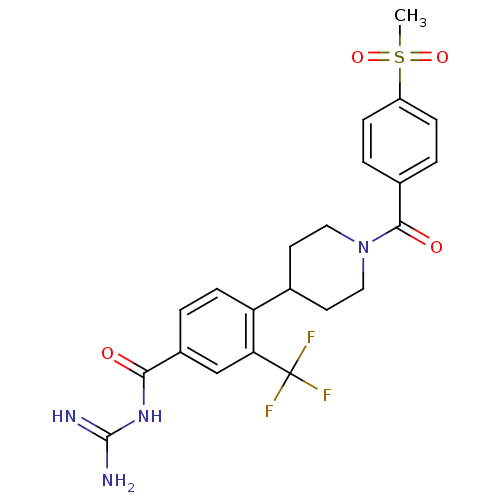 (CHEMBL2170632) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of NHE1 in human HT-29 cells assessed as intracellular pH change after 30 mins | J Med Chem 55: 7114-40 (2012) Article DOI: 10.1021/jm300601d BindingDB Entry DOI: 10.7270/Q2FB5430 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50396443 (CHEMBL2170632) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibiton of CYP2C9 in human liver microsomes using 7-methoxy-4-(trifluoromethyl)-coumarin as substrate after 45 mins by LC/MS/MS analysis | J Med Chem 55: 7114-40 (2012) Article DOI: 10.1021/jm300601d BindingDB Entry DOI: 10.7270/Q2FB5430 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50396443 (CHEMBL2170632) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibiton of CYP2D6 in human liver microsomes using 3-[2-(N,N-diethyl-N-methylamino)ethyl]-7- methoxy-4-methylcoumarin as substrate after 45 mins by ... | J Med Chem 55: 7114-40 (2012) Article DOI: 10.1021/jm300601d BindingDB Entry DOI: 10.7270/Q2FB5430 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/hydrogen exchanger 1 (Homo sapiens (Human)) | BDBM50396443 (CHEMBL2170632) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 93 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of NHE1 in human platelet rich plasma assessed as reduction of propionate medium induced platelet swelling measured every 6 secs for 5 min... | J Med Chem 55: 7114-40 (2012) Article DOI: 10.1021/jm300601d BindingDB Entry DOI: 10.7270/Q2FB5430 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50396443 (CHEMBL2170632) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibiton of CYP3A4 in human liver microsomes using 7-benzyloxy-4-(trifluoromethyl)-coumarin as substrate after 30 mins by LC/MS/MS analysis | J Med Chem 55: 7114-40 (2012) Article DOI: 10.1021/jm300601d BindingDB Entry DOI: 10.7270/Q2FB5430 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C19 (Homo sapiens (Human)) | BDBM50396443 (CHEMBL2170632) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibiton of CYP2C19 in human liver microsomes using 7-ethoxy-3-cyanocoumarin as substrate after 45 mins by LC/MS/MS analysis | J Med Chem 55: 7114-40 (2012) Article DOI: 10.1021/jm300601d BindingDB Entry DOI: 10.7270/Q2FB5430 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||