 Found 5 hits for monomerid = 50398089
Found 5 hits for monomerid = 50398089 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Lysophosphatidic acid receptor 1
(Homo sapiens (Human)) | BDBM50398089
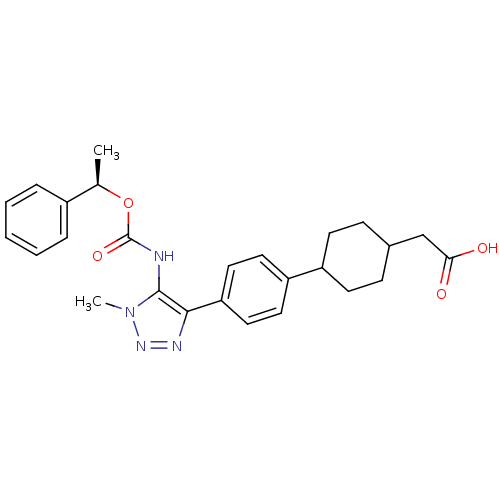
(CHEMBL2182051 | US9321738, 14)Show SMILES C[C@@H](OC(=O)Nc1c(nnn1C)-c1ccc(cc1)C1CCC(CC(O)=O)CC1)c1ccccc1 |r,wD:1.0,(51.8,-50.88,;51.04,-52.22,;49.5,-52.23,;48.72,-50.9,;49.48,-49.56,;47.18,-50.91,;46.4,-49.58,;44.94,-49.14,;44.9,-47.61,;46.34,-47.1,;47.28,-48.32,;48.82,-48.29,;43.57,-49.86,;43.51,-51.4,;42.15,-52.11,;40.85,-51.28,;40.92,-49.74,;42.28,-49.03,;39.48,-51.99,;39.42,-53.53,;38.05,-54.24,;36.75,-53.41,;35.38,-54.12,;34.08,-53.29,;32.71,-54,;34.15,-51.75,;36.82,-51.86,;38.19,-51.16,;51.82,-53.55,;51.06,-54.88,;51.83,-56.21,;53.38,-56.2,;54.14,-54.85,;53.35,-53.52,)| Show InChI InChI=1S/C26H30N4O4/c1-17(19-6-4-3-5-7-19)34-26(33)27-25-24(28-29-30(25)2)22-14-12-21(13-15-22)20-10-8-18(9-11-20)16-23(31)32/h3-7,12-15,17-18,20H,8-11,16H2,1-2H3,(H,27,33)(H,31,32)/t17-,18?,20?/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 58 | n/a | n/a | n/a | n/a | n/a | 25 |
HOFFMAN-LA ROCHE INC.
US Patent
| Assay Description
Test compounds were prepared by adding 90 μL of HBSS/20 mM HEPES/0.1% BSA buffer to 2 μL of serially diluted compounds. To prepare serial dilut... |
US Patent US9321738 (2016)
BindingDB Entry DOI: 10.7270/Q2B56HKJ |
More data for this
Ligand-Target Pair | |
Lysophosphatidic acid receptor 3
(Homo sapiens (Human)) | BDBM50398089

(CHEMBL2182051 | US9321738, 14)Show SMILES C[C@@H](OC(=O)Nc1c(nnn1C)-c1ccc(cc1)C1CCC(CC(O)=O)CC1)c1ccccc1 |r,wD:1.0,(51.8,-50.88,;51.04,-52.22,;49.5,-52.23,;48.72,-50.9,;49.48,-49.56,;47.18,-50.91,;46.4,-49.58,;44.94,-49.14,;44.9,-47.61,;46.34,-47.1,;47.28,-48.32,;48.82,-48.29,;43.57,-49.86,;43.51,-51.4,;42.15,-52.11,;40.85,-51.28,;40.92,-49.74,;42.28,-49.03,;39.48,-51.99,;39.42,-53.53,;38.05,-54.24,;36.75,-53.41,;35.38,-54.12,;34.08,-53.29,;32.71,-54,;34.15,-51.75,;36.82,-51.86,;38.19,-51.16,;51.82,-53.55,;51.06,-54.88,;51.83,-56.21,;53.38,-56.2,;54.14,-54.85,;53.35,-53.52,)| Show InChI InChI=1S/C26H30N4O4/c1-17(19-6-4-3-5-7-19)34-26(33)27-25-24(28-29-30(25)2)22-14-12-21(13-15-22)20-10-8-18(9-11-20)16-23(31)32/h3-7,12-15,17-18,20H,8-11,16H2,1-2H3,(H,27,33)(H,31,32)/t17-,18?,20?/m1/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 1.86E+4 | n/a | n/a | n/a | n/a | n/a | 25 |
HOFFMAN-LA ROCHE INC.
US Patent
| Assay Description
Test compounds were prepared by adding 90 μL of HBSS/20 mM HEPES/0.1% BSA buffer to 2 μL of serially diluted compounds. To prepare serial dilut... |
US Patent US9321738 (2016)
BindingDB Entry DOI: 10.7270/Q2B56HKJ |
More data for this
Ligand-Target Pair | |
Lysophosphatidic acid receptor 1
(Homo sapiens (Human)) | BDBM50398089

(CHEMBL2182051 | US9321738, 14)Show SMILES C[C@@H](OC(=O)Nc1c(nnn1C)-c1ccc(cc1)C1CCC(CC(O)=O)CC1)c1ccccc1 |r,wD:1.0,(51.8,-50.88,;51.04,-52.22,;49.5,-52.23,;48.72,-50.9,;49.48,-49.56,;47.18,-50.91,;46.4,-49.58,;44.94,-49.14,;44.9,-47.61,;46.34,-47.1,;47.28,-48.32,;48.82,-48.29,;43.57,-49.86,;43.51,-51.4,;42.15,-52.11,;40.85,-51.28,;40.92,-49.74,;42.28,-49.03,;39.48,-51.99,;39.42,-53.53,;38.05,-54.24,;36.75,-53.41,;35.38,-54.12,;34.08,-53.29,;32.71,-54,;34.15,-51.75,;36.82,-51.86,;38.19,-51.16,;51.82,-53.55,;51.06,-54.88,;51.83,-56.21,;53.38,-56.2,;54.14,-54.85,;53.35,-53.52,)| Show InChI InChI=1S/C26H30N4O4/c1-17(19-6-4-3-5-7-19)34-26(33)27-25-24(28-29-30(25)2)22-14-12-21(13-15-22)20-10-8-18(9-11-20)16-23(31)32/h3-7,12-15,17-18,20H,8-11,16H2,1-2H3,(H,27,33)(H,31,32)/t17-,18?,20?/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 58 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche
Curated by ChEMBL
| Assay Description
Antagonist activity at human recombinant LPA1 expressed in chem-1 cells assessed as inhibition of LPA-induced intracellular calcium mobilization incu... |
J Med Chem 55: 7920-39 (2012)
Article DOI: 10.1021/jm301022v
BindingDB Entry DOI: 10.7270/Q26974Q9 |
More data for this
Ligand-Target Pair | |
Lysophosphatidic acid receptor 3
(Homo sapiens (Human)) | BDBM50398089

(CHEMBL2182051 | US9321738, 14)Show SMILES C[C@@H](OC(=O)Nc1c(nnn1C)-c1ccc(cc1)C1CCC(CC(O)=O)CC1)c1ccccc1 |r,wD:1.0,(51.8,-50.88,;51.04,-52.22,;49.5,-52.23,;48.72,-50.9,;49.48,-49.56,;47.18,-50.91,;46.4,-49.58,;44.94,-49.14,;44.9,-47.61,;46.34,-47.1,;47.28,-48.32,;48.82,-48.29,;43.57,-49.86,;43.51,-51.4,;42.15,-52.11,;40.85,-51.28,;40.92,-49.74,;42.28,-49.03,;39.48,-51.99,;39.42,-53.53,;38.05,-54.24,;36.75,-53.41,;35.38,-54.12,;34.08,-53.29,;32.71,-54,;34.15,-51.75,;36.82,-51.86,;38.19,-51.16,;51.82,-53.55,;51.06,-54.88,;51.83,-56.21,;53.38,-56.2,;54.14,-54.85,;53.35,-53.52,)| Show InChI InChI=1S/C26H30N4O4/c1-17(19-6-4-3-5-7-19)34-26(33)27-25-24(28-29-30(25)2)22-14-12-21(13-15-22)20-10-8-18(9-11-20)16-23(31)32/h3-7,12-15,17-18,20H,8-11,16H2,1-2H3,(H,27,33)(H,31,32)/t17-,18?,20?/m1/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.86E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche
Curated by ChEMBL
| Assay Description
Antagonist activity at human recombinant LPA3 expressed in chem-1 cells assessed as inhibition of LPA-induced intracellular calcium mobilization incu... |
J Med Chem 55: 7920-39 (2012)
Article DOI: 10.1021/jm301022v
BindingDB Entry DOI: 10.7270/Q26974Q9 |
More data for this
Ligand-Target Pair | |
Lysophosphatidic acid receptor 1
(Homo sapiens (Human)) | BDBM50398089

(CHEMBL2182051 | US9321738, 14)Show SMILES C[C@@H](OC(=O)Nc1c(nnn1C)-c1ccc(cc1)C1CCC(CC(O)=O)CC1)c1ccccc1 |r,wD:1.0,(51.8,-50.88,;51.04,-52.22,;49.5,-52.23,;48.72,-50.9,;49.48,-49.56,;47.18,-50.91,;46.4,-49.58,;44.94,-49.14,;44.9,-47.61,;46.34,-47.1,;47.28,-48.32,;48.82,-48.29,;43.57,-49.86,;43.51,-51.4,;42.15,-52.11,;40.85,-51.28,;40.92,-49.74,;42.28,-49.03,;39.48,-51.99,;39.42,-53.53,;38.05,-54.24,;36.75,-53.41,;35.38,-54.12,;34.08,-53.29,;32.71,-54,;34.15,-51.75,;36.82,-51.86,;38.19,-51.16,;51.82,-53.55,;51.06,-54.88,;51.83,-56.21,;53.38,-56.2,;54.14,-54.85,;53.35,-53.52,)| Show InChI InChI=1S/C26H30N4O4/c1-17(19-6-4-3-5-7-19)34-26(33)27-25-24(28-29-30(25)2)22-14-12-21(13-15-22)20-10-8-18(9-11-20)16-23(31)32/h3-7,12-15,17-18,20H,8-11,16H2,1-2H3,(H,27,33)(H,31,32)/t17-,18?,20?/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche
Curated by ChEMBL
| Assay Description
Antagonist activity at LPA1 in human lung fibroblasts assessed as inhibition of LPA-induced contraction after 18 hrs by 3D collagen gel contraction a... |
J Med Chem 55: 7920-39 (2012)
Article DOI: 10.1021/jm301022v
BindingDB Entry DOI: 10.7270/Q26974Q9 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data




