

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50398112 CHEMBL2182045::US9321738, 7
SMILES: CC(C)[C@@H](C)OC(=O)Nc1c(nnn1C)-c1ccc(cc1)-c1ccc(cc1)C1(CC1)C(O)=O
InChI Key: InChIKey=GTLJBSMYSBXGGC-MRXNPFEDSA-N
Data: 3 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lysophosphatidic acid receptor 1 (Homo sapiens (Human)) | BDBM50398112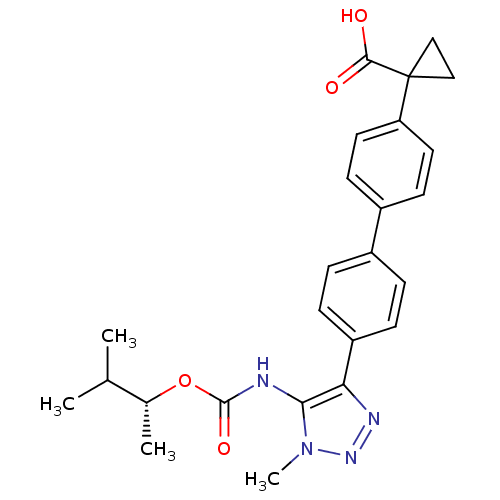 (CHEMBL2182045 | US9321738, 7) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | 25 |
HOFFMAN-LA ROCHE INC. US Patent | Assay Description Test compounds were prepared by adding 90 μL of HBSS/20 mM HEPES/0.1% BSA buffer to 2 μL of serially diluted compounds. To prepare serial dilut... | US Patent US9321738 (2016) BindingDB Entry DOI: 10.7270/Q2B56HKJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysophosphatidic acid receptor 1 (Homo sapiens (Human)) | BDBM50398112 (CHEMBL2182045 | US9321738, 7) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Curated by ChEMBL | Assay Description Antagonist activity at human recombinant LPA1 expressed in chem-1 cells assessed as inhibition of LPA-induced intracellular calcium mobilization incu... | J Med Chem 55: 7920-39 (2012) Article DOI: 10.1021/jm301022v BindingDB Entry DOI: 10.7270/Q26974Q9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysophosphatidic acid receptor 3 (Homo sapiens (Human)) | BDBM50398112 (CHEMBL2182045 | US9321738, 7) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | 25 |
HOFFMAN-LA ROCHE INC. US Patent | Assay Description Test compounds were prepared by adding 90 μL of HBSS/20 mM HEPES/0.1% BSA buffer to 2 μL of serially diluted compounds. To prepare serial dilut... | US Patent US9321738 (2016) BindingDB Entry DOI: 10.7270/Q2B56HKJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||