

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50398725 CHEMBL2179249::US10227295, Compound 5b::US9409858, 5b::US9751832, Compound 5b::US9956192, Compound 5b
SMILES: COc1ccccc1NC(=O)N(CCCO)Cc1ccc(cc1)C(=O)NO
InChI Key: InChIKey=MQGIMYGUJWRQDY-UHFFFAOYSA-N
Data: 10 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50398725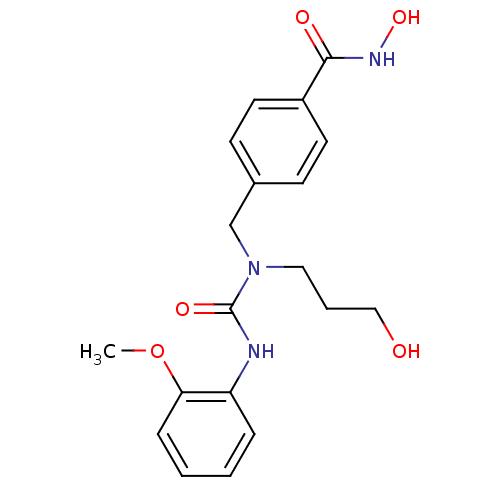 (CHEMBL2179249 | US10227295, Compound 5b | US940985...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.91E+3 | n/a | n/a | n/a | n/a | 8.0 | n/a |
H. Lee Moffitt Cancer Center and Research Institute, Inc.; Board of Trustees of the University of Illinois US Patent | Assay Description HDAC inhibition assays were performed by Reaction Biology Corp. (Malvern, Pa.) using isolated human, recombinant full-length HDAC1 and -6 from a bacu... | US Patent US9409858 (2016) BindingDB Entry DOI: 10.7270/Q21V5CWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cereblon/Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50398725 (CHEMBL2179249 | US10227295, Compound 5b | US940985...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.38 | n/a | n/a | n/a | n/a | 8.0 | n/a |
H. Lee Moffitt Cancer Center and Research Institute, Inc.; Board of Trustees of the University of Illinois US Patent | Assay Description HDAC inhibition assays were performed by Reaction Biology Corp. (Malvern, Pa.) using isolated human, recombinant full-length HDAC1 and -6 from a bacu... | US Patent US9409858 (2016) BindingDB Entry DOI: 10.7270/Q21V5CWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cereblon/Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50398725 (CHEMBL2179249 | US10227295, Compound 5b | US940985...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.38 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago Curated by ChEMBL | Assay Description Inhibition of human recombinant HDAC6 expressed in Sf9 cells incubated for 2 hrs using RHKK-Ac fluorogenic substrate | J Med Chem 55: 9891-9 (2012) Article DOI: 10.1021/jm301098e BindingDB Entry DOI: 10.7270/Q2WQ04XP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50398725 (CHEMBL2179249 | US10227295, Compound 5b | US940985...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.91E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago Curated by ChEMBL | Assay Description Inhibition of human recombinant HDAC1 expressed in Sf9 cells incubated for 2 hrs using RHKK-Ac fluorogenic substrate | J Med Chem 55: 9891-9 (2012) Article DOI: 10.1021/jm301098e BindingDB Entry DOI: 10.7270/Q2WQ04XP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cereblon/Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50398725 (CHEMBL2179249 | US10227295, Compound 5b | US940985...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.38 | n/a | n/a | n/a | n/a | n/a | n/a |
KuDOS Pharmaceuticals Ltd. | Assay Description HDAC inhibition assays were performed by Reaction Biology Corp. (Malvern, Pa.) using isolated human, recombinant full-length HDAC1 and -6 from a bacu... | J Med Chem 51: 6581-91 (2008) BindingDB Entry DOI: 10.7270/Q2BZ68CW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cereblon/Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50398725 (CHEMBL2179249 | US10227295, Compound 5b | US940985...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.38 | n/a | n/a | n/a | n/a | n/a | n/a |
H. Lee Moffitt Cancer Center and Research Institute, Inc.; Board of Trustees of the University of Illinois US Patent | Assay Description HDAC inhibition assays were performed by Reaction Biology Corp. (Malvern, Pa.) using isolated human, recombinant full-length HDAC1 and -6 from a bacu... | US Patent US9751832 (2017) BindingDB Entry DOI: 10.7270/Q23T9KBR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50398725 (CHEMBL2179249 | US10227295, Compound 5b | US940985...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.91E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis (R&D) Ltd | Assay Description HDAC inhibition assays were performed by Reaction Biology Corp. (Malvern, Pa.) using isolated human, recombinant full-length HDAC1 and -6 from a bacu... | J Med Chem 51: 196-218 (2008) BindingDB Entry DOI: 10.7270/Q2VD71RF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cereblon/Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50398725 (CHEMBL2179249 | US10227295, Compound 5b | US940985...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.38 | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis (R&D) Ltd | Assay Description HDAC inhibition assays were performed by Reaction Biology Corp. (Malvern, Pa.) using isolated human, recombinant full-length HDAC1 and -6 from a bacu... | J Med Chem 51: 196-218 (2008) BindingDB Entry DOI: 10.7270/Q2VD71RF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50398725 (CHEMBL2179249 | US10227295, Compound 5b | US940985...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.91E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
KuDOS Pharmaceuticals Ltd. | Assay Description HDAC inhibition assays were performed by Reaction Biology Corp. (Malvern, Pa.) using isolated human, recombinant full-length HDAC1 and -6 from a bacu... | J Med Chem 51: 6581-91 (2008) BindingDB Entry DOI: 10.7270/Q2BZ68CW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50398725 (CHEMBL2179249 | US10227295, Compound 5b | US940985...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.91E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
H. Lee Moffitt Cancer Center and Research Institute, Inc.; Board of Trustees of the University of Illinois US Patent | Assay Description HDAC inhibition assays were performed by Reaction Biology Corp. (Malvern, Pa.) using isolated human, recombinant full-length HDAC1 and -6 from a bacu... | US Patent US9751832 (2017) BindingDB Entry DOI: 10.7270/Q23T9KBR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||