

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50399031 CHEMBL2178257::US8637526, 250
SMILES: Nc1ccn2ncc(C(=O)Nc3conc3-c3cccc(Cl)c3)c2n1
InChI Key: InChIKey=LVTOASRSQVQBSU-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tyrosine-protein kinase JAK2 (Homo sapiens (Human)) | BDBM50399031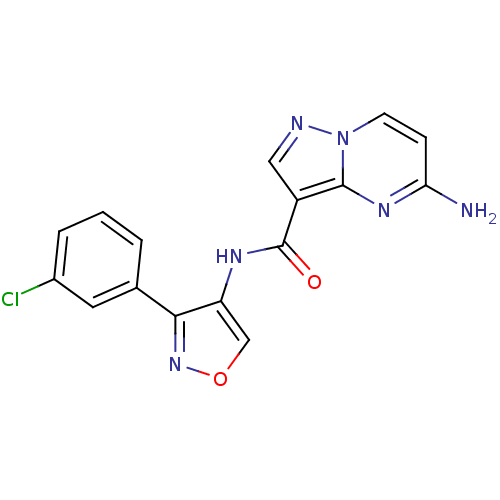 (CHEMBL2178257 | US8637526, 250) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Inhibition of purified JAK2 incubated for 30 mins | J Med Chem 55: 10090-107 (2012) Article DOI: 10.1021/jm3012239 BindingDB Entry DOI: 10.7270/Q2Q241D4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK2 (Homo sapiens (Human)) | BDBM50399031 (CHEMBL2178257 | US8637526, 250) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 2 | -11.7 | n/a | n/a | n/a | n/a | n/a | 7.2 | 22 |
Genentech, Inc. US Patent | Assay Description To determine the inhibition constants (Ki), compounds were diluted serially in DMSO and added to 50 kinase reactions containing 0.2 nM purified JAK2 ... | US Patent US8637526 (2014) BindingDB Entry DOI: 10.7270/Q25719Q3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM50399031 (CHEMBL2178257 | US8637526, 250) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 28.4 | n/a | n/a | n/a | n/a | n/a | n/a | 7.2 | n/a |
Genentech, Inc. US Patent | Assay Description To determine inhibition constants (Ki), compounds were diluted serially in DMSO and added to 50 uL kinase reactions containing 1.5 nM JAK1, 0.2 nM pu... | US Patent US8637526 (2014) BindingDB Entry DOI: 10.7270/Q25719Q3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Non-receptor tyrosine-protein kinase TYK2 (Homo sapiens (Human)) | BDBM50399031 (CHEMBL2178257 | US8637526, 250) | PDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 36.1 | n/a | n/a | n/a | n/a | n/a | n/a | 7.2 | n/a |
Genentech, Inc. US Patent | Assay Description To determine inhibition constants (Ki), compounds were diluted serially in DMSO and added to 50 uL kinase reactions containing 1.5 nM JAK1, 0.2 nM pu... | US Patent US8637526 (2014) BindingDB Entry DOI: 10.7270/Q25719Q3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM50399031 (CHEMBL2178257 | US8637526, 250) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 55.6 | -9.79 | n/a | n/a | n/a | n/a | n/a | 7.2 | 22 |
Genentech, Inc. US Patent | Assay Description To determine inhibition constants (Ki), compounds were diluted serially in DMSO and added to 50 uL kinase reactions containing 5 nM purified JAK3 enz... | US Patent US8637526 (2014) BindingDB Entry DOI: 10.7270/Q25719Q3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A (Homo sapiens (Human)) | BDBM50399031 (CHEMBL2178257 | US8637526, 250) | PDB MMDB Reactome pathway KEGG B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Inhibition of CYP1A2 | J Med Chem 55: 10090-107 (2012) Article DOI: 10.1021/jm3012239 BindingDB Entry DOI: 10.7270/Q2Q241D4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||