

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50399331 CHEMBL2180882::US9364459, C
SMILES: COc1cc(cc(OC)c1OC)C1=C(C(=O)NC1=O)c1c[nH]c2cnccc12
InChI Key: InChIKey=CHCLWGWWJCQQCV-UHFFFAOYSA-N
Data: 6 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM50399331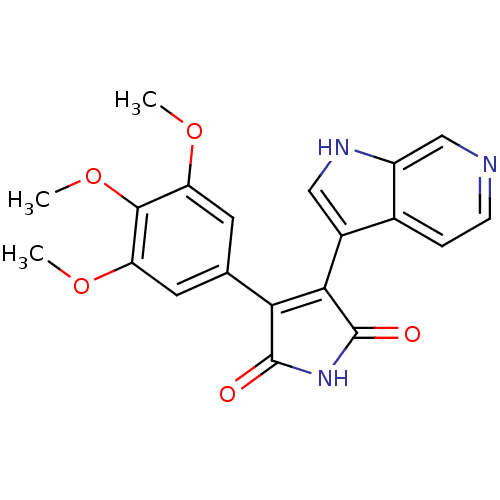 (CHEMBL2180882 | US9364459, C) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
Johannes Gutenberg-Universität Mainz; Universitätsmedizin der Johannes Gutenberg-Universität Mainz US Patent | Assay Description The effect of test compounds on the activity of the protein kinases GSK-3β, VEGFR-2 and FLT-3 was evaluated based on half maximal inhibitory con... | US Patent US9364459 (2016) BindingDB Entry DOI: 10.7270/Q2ZK5FK2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM50399331 (CHEMBL2180882 | US9364459, C) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johannes Gutenberg-Universität Mainz; Universitätsmedizin der Johannes Gutenberg-Universität Mainz US Patent | Assay Description The effect of test compounds on the activity of the protein kinases GSK-3β, VEGFR-2 and FLT-3 was evaluated based on half maximal inhibitory con... | US Patent US9364459 (2016) BindingDB Entry DOI: 10.7270/Q2ZK5FK2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM50399331 (CHEMBL2180882 | US9364459, C) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
Johannes Gutenberg University of Mainz Curated by ChEMBL | Assay Description Inhibition of VEGFR2 | J Med Chem 55: 9531-40 (2012) Article DOI: 10.1021/jm301217c BindingDB Entry DOI: 10.7270/Q21C1Z0P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM50399331 (CHEMBL2180882 | US9364459, C) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Johannes Gutenberg University of Mainz Curated by ChEMBL | Assay Description Inhibition of GSK3beta | J Med Chem 55: 9531-40 (2012) Article DOI: 10.1021/jm301217c BindingDB Entry DOI: 10.7270/Q21C1Z0P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM50399331 (CHEMBL2180882 | US9364459, C) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Johannes Gutenberg University of Mainz Curated by ChEMBL | Assay Description Inhibition of FLT3 | J Med Chem 55: 9531-40 (2012) Article DOI: 10.1021/jm301217c BindingDB Entry DOI: 10.7270/Q21C1Z0P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM50399331 (CHEMBL2180882 | US9364459, C) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
Johannes Gutenberg-Universität Mainz; Universitätsmedizin der Johannes Gutenberg-Universität Mainz US Patent | Assay Description The effect of test compounds on the activity of the protein kinases GSK-3β, VEGFR-2 and FLT-3 was evaluated based on half maximal inhibitory con... | US Patent US9364459 (2016) BindingDB Entry DOI: 10.7270/Q2ZK5FK2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||