 Found 8 hits for monomerid = 50400621
Found 8 hits for monomerid = 50400621 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Protein-glutamine gamma-glutamyltransferase
(Homo sapiens (Human)) | BDBM50400621
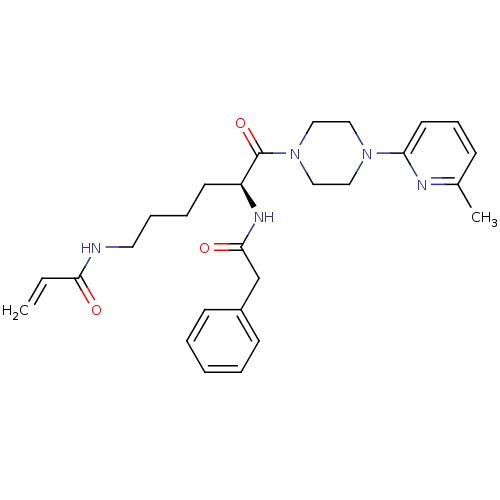
(CHEMBL2203451)Show SMILES Cc1cccc(n1)N1CCN(CC1)C(=O)[C@H](CCCCNC(=O)C=C)NC(=O)Cc1ccccc1 |r| Show InChI InChI=1S/C27H35N5O3/c1-3-25(33)28-15-8-7-13-23(30-26(34)20-22-11-5-4-6-12-22)27(35)32-18-16-31(17-19-32)24-14-9-10-21(2)29-24/h3-6,9-12,14,23H,1,7-8,13,15-20H2,2H3,(H,28,33)(H,30,34)/t23-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.73E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Helmholtz-Zentrum Dresden-Rossendorf
Curated by ChEMBL
| Assay Description
Reversible inhibition of human TGase 2 using Z-Glu(HMC)-Gly-OH as substrate measured at 20 secs interval over 900 secs by fluorimetric assay |
J Med Chem 61: 4528-4560 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00286
BindingDB Entry DOI: 10.7270/Q2W66PC7 |
More data for this
Ligand-Target Pair | |
Coagulation factor XIII
(Homo sapiens (Human)) | BDBM50400621

(CHEMBL2203451)Show SMILES Cc1cccc(n1)N1CCN(CC1)C(=O)[C@H](CCCCNC(=O)C=C)NC(=O)Cc1ccccc1 |r| Show InChI InChI=1S/C27H35N5O3/c1-3-25(33)28-15-8-7-13-23(30-26(34)20-22-11-5-4-6-12-22)27(35)32-18-16-31(17-19-32)24-14-9-10-21(2)29-24/h3-6,9-12,14,23H,1,7-8,13,15-20H2,2H3,(H,28,33)(H,30,34)/t23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of coagulation factor 13a |
ACS Med Chem Lett 3: 1024-1028 (2012)
Article DOI: 10.1021/ml300241m
BindingDB Entry DOI: 10.7270/Q2TB1823 |
More data for this
Ligand-Target Pair | |
Transglutaminase-6 (TG6)
(Homo sapiens (Human)) | BDBM50400621

(CHEMBL2203451)Show SMILES Cc1cccc(n1)N1CCN(CC1)C(=O)[C@H](CCCCNC(=O)C=C)NC(=O)Cc1ccccc1 |r| Show InChI InChI=1S/C27H35N5O3/c1-3-25(33)28-15-8-7-13-23(30-26(34)20-22-11-5-4-6-12-22)27(35)32-18-16-31(17-19-32)24-14-9-10-21(2)29-24/h3-6,9-12,14,23H,1,7-8,13,15-20H2,2H3,(H,28,33)(H,30,34)/t23-/m0/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >8.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of transglutaminase 6 |
ACS Med Chem Lett 3: 1024-1028 (2012)
Article DOI: 10.1021/ml300241m
BindingDB Entry DOI: 10.7270/Q2TB1823 |
More data for this
Ligand-Target Pair | |
Protein-glutamine gamma-glutamyltransferase
(Homo sapiens (Human)) | BDBM50400621

(CHEMBL2203451)Show SMILES Cc1cccc(n1)N1CCN(CC1)C(=O)[C@H](CCCCNC(=O)C=C)NC(=O)Cc1ccccc1 |r| Show InChI InChI=1S/C27H35N5O3/c1-3-25(33)28-15-8-7-13-23(30-26(34)20-22-11-5-4-6-12-22)27(35)32-18-16-31(17-19-32)24-14-9-10-21(2)29-24/h3-6,9-12,14,23H,1,7-8,13,15-20H2,2H3,(H,28,33)(H,30,34)/t23-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 790 | n/a | n/a | n/a | n/a | n/a | n/a |
Helmholtz-Zentrum Dresden-Rossendorf
Curated by ChEMBL
| Assay Description
Inhibition of TGase 2 in human A375 cells using R-I-Cad/DMC as substrate preincubated for 5 mins followed by substrate addition measured at 30 secs i... |
J Med Chem 61: 4528-4560 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00286
BindingDB Entry DOI: 10.7270/Q2W66PC7 |
More data for this
Ligand-Target Pair | |
Transglutaminase-1 (TG1)
(Homo sapiens (Human)) | BDBM50400621

(CHEMBL2203451)Show SMILES Cc1cccc(n1)N1CCN(CC1)C(=O)[C@H](CCCCNC(=O)C=C)NC(=O)Cc1ccccc1 |r| Show InChI InChI=1S/C27H35N5O3/c1-3-25(33)28-15-8-7-13-23(30-26(34)20-22-11-5-4-6-12-22)27(35)32-18-16-31(17-19-32)24-14-9-10-21(2)29-24/h3-6,9-12,14,23H,1,7-8,13,15-20H2,2H3,(H,28,33)(H,30,34)/t23-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of transglutaminase 1 |
ACS Med Chem Lett 3: 1024-1028 (2012)
Article DOI: 10.1021/ml300241m
BindingDB Entry DOI: 10.7270/Q2TB1823 |
More data for this
Ligand-Target Pair | |
Protein-glutamine gamma-glutamyltransferase
(Homo sapiens (Human)) | BDBM50400621

(CHEMBL2203451)Show SMILES Cc1cccc(n1)N1CCN(CC1)C(=O)[C@H](CCCCNC(=O)C=C)NC(=O)Cc1ccccc1 |r| Show InChI InChI=1S/C27H35N5O3/c1-3-25(33)28-15-8-7-13-23(30-26(34)20-22-11-5-4-6-12-22)27(35)32-18-16-31(17-19-32)24-14-9-10-21(2)29-24/h3-6,9-12,14,23H,1,7-8,13,15-20H2,2H3,(H,28,33)(H,30,34)/t23-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 310 | n/a | n/a | n/a | n/a | n/a | n/a |
Helmholtz-Zentrum Dresden-Rossendorf
Curated by ChEMBL
| Assay Description
Inhibition of human TGase 2 using R-I-Cad/DMC as substrate preincubated for 5 mins followed by substrate addition measured at 30 secs interval over 9... |
J Med Chem 61: 4528-4560 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00286
BindingDB Entry DOI: 10.7270/Q2W66PC7 |
More data for this
Ligand-Target Pair | |
Protein-glutamine gamma-glutamyltransferase 2 (TG2)
(Homo sapiens (Human)) | BDBM50400621

(CHEMBL2203451)Show SMILES Cc1cccc(n1)N1CCN(CC1)C(=O)[C@H](CCCCNC(=O)C=C)NC(=O)Cc1ccccc1 |r| Show InChI InChI=1S/C27H35N5O3/c1-3-25(33)28-15-8-7-13-23(30-26(34)20-22-11-5-4-6-12-22)27(35)32-18-16-31(17-19-32)24-14-9-10-21(2)29-24/h3-6,9-12,14,23H,1,7-8,13,15-20H2,2H3,(H,28,33)(H,30,34)/t23-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Irreversible inhibition of transglutaminase 2 |
ACS Med Chem Lett 3: 1024-1028 (2012)
Article DOI: 10.1021/ml300241m
BindingDB Entry DOI: 10.7270/Q2TB1823 |
More data for this
Ligand-Target Pair | |
Transglutaminase-3 (TG3)
(Homo sapiens (Human)) | BDBM50400621

(CHEMBL2203451)Show SMILES Cc1cccc(n1)N1CCN(CC1)C(=O)[C@H](CCCCNC(=O)C=C)NC(=O)Cc1ccccc1 |r| Show InChI InChI=1S/C27H35N5O3/c1-3-25(33)28-15-8-7-13-23(30-26(34)20-22-11-5-4-6-12-22)27(35)32-18-16-31(17-19-32)24-14-9-10-21(2)29-24/h3-6,9-12,14,23H,1,7-8,13,15-20H2,2H3,(H,28,33)(H,30,34)/t23-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >8.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of transglutaminase 3 |
ACS Med Chem Lett 3: 1024-1028 (2012)
Article DOI: 10.1021/ml300241m
BindingDB Entry DOI: 10.7270/Q2TB1823 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data