

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM482159 BDBM50401284::GSK2578215A
SMILES: Fc1cc(ccn1)-c1ccc(OCc2ccccc2)c(c1)C(=O)Nc1cccnc1
InChI Key: InChIKey=WCIGMFCFPXZRMQ-UHFFFAOYSA-N
Data: 7 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Leucine-rich repeat serine/threonine-protein kinase 2 (LRRK2) (Homo sapiens (Human)) | BDBM482159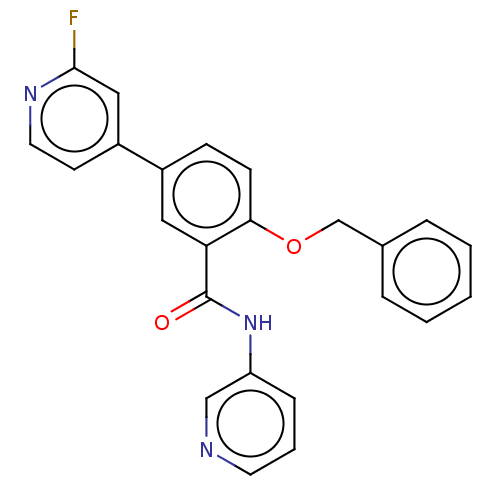 (BDBM50401284 | GSK2578215A) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 10.9 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals R&D Curated by ChEMBL | Assay Description Inhibition of recombinant His6-Tev-tagged LRRK2 expressed in baculovirus assessed as inhibition of LRRKtide phospohorylation by HTRF assay | Bioorg Med Chem Lett 22: 5625-9 (2012) Article DOI: 10.1016/j.bmcl.2012.06.104 BindingDB Entry DOI: 10.7270/Q2BZ6767 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine-rich repeat serine/threonine-protein kinase 2 (LRRK2) (Homo sapiens (Human)) | BDBM482159 (BDBM50401284 | GSK2578215A) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Charles River Curated by ChEMBL | Assay Description Inhibition of full length wild-type LRRK2 (unknown origin) using biotinylated ezrin/radaxin/meosin peptide as substrate measured after 1 hr | Bioorg Med Chem Lett 27: 2520-2527 (2017) Article DOI: 10.1016/j.bmcl.2017.03.098 BindingDB Entry DOI: 10.7270/Q2474D0R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine-rich repeat serine/threonine-protein kinase 2 (LRRK2) (Homo sapiens (Human)) | BDBM482159 (BDBM50401284 | GSK2578215A) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Neurodegeneration DPU, Neurosciences Therapeutic Area Unit, GSK Pharmaceuticals R&D, 898 Halei Road, Zhangjiang Hi-Tech Park, Pudong, Shanghai 201203, PR China. Curated by ChEMBL | Assay Description Inhibition of LRRK2 (unknown origin) by HTRF assay | Bioorg Med Chem Lett 27: 4034-4038 (2017) Article DOI: 10.1016/j.bmcl.2017.07.052 BindingDB Entry DOI: 10.7270/Q2MG7S1N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GST-LRRK2 (aa 1326-2527,A2016T,G2019S) (Homo sapiens (Human)) | BDBM482159 (BDBM50401284 | GSK2578215A) | PDB MMDB B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | US Patent | n/a | n/a | 61.3 | n/a | n/a | n/a | n/a | n/a | n/a |
DANA-FARBER CANCER INSTITUTE, INC. US Patent | Assay Description Active GST-LRRK2 (1326-2527), GST-LRRK2[G2019S] (1326-2527), GST-LRRK2[A2016T] (1326-2527) and GST-LRRK2[A2016T+G2019S] (1326-2527) enzyme was purifi... | US Patent US10913744 (2021) | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GST-LRRK2 (aa 1326-2527) (Homo sapiens (Human)) | BDBM482159 (BDBM50401284 | GSK2578215A) | PDB GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | US Patent | n/a | n/a | 10.9 | n/a | n/a | n/a | n/a | n/a | n/a |
DANA-FARBER CANCER INSTITUTE, INC. US Patent | Assay Description Active GST-LRRK2 (1326-2527), GST-LRRK2[G2019S] (1326-2527), GST-LRRK2[A2016T] (1326-2527) and GST-LRRK2[A2016T+G2019S] (1326-2527) enzyme was purifi... | US Patent US10913744 (2021) | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GST-LRRK2 (aa 1326-2527,A2016T) (Homo sapiens (Human)) | BDBM482159 (BDBM50401284 | GSK2578215A) | PDB MMDB GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | US Patent | n/a | n/a | 81.1 | n/a | n/a | n/a | n/a | n/a | n/a |
DANA-FARBER CANCER INSTITUTE, INC. US Patent | Assay Description Active GST-LRRK2 (1326-2527), GST-LRRK2[G2019S] (1326-2527), GST-LRRK2[A2016T] (1326-2527) and GST-LRRK2[A2016T+G2019S] (1326-2527) enzyme was purifi... | US Patent US10913744 (2021) | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GST-LRRK2 (aa 1326-2527,G2019S) (Homo sapiens (Human)) | BDBM482159 (BDBM50401284 | GSK2578215A) | PDB GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | US Patent | n/a | n/a | 8.90 | n/a | n/a | n/a | n/a | n/a | n/a |
DANA-FARBER CANCER INSTITUTE, INC. US Patent | Assay Description Active GST-LRRK2 (1326-2527), GST-LRRK2[G2019S] (1326-2527), GST-LRRK2[A2016T] (1326-2527) and GST-LRRK2[A2016T+G2019S] (1326-2527) enzyme was purifi... | US Patent US10913744 (2021) | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||