 Found 9 hits for monomerid = 50404028
Found 9 hits for monomerid = 50404028 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50404028
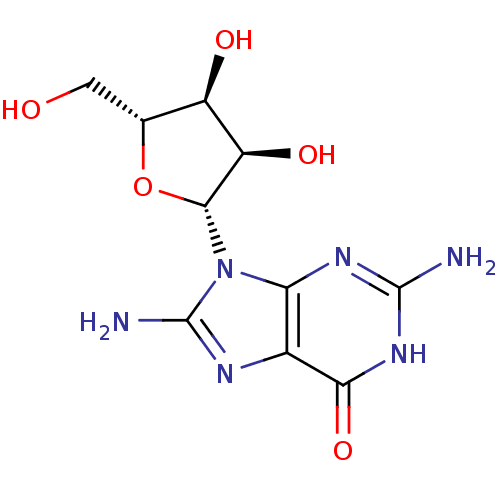
(CHEMBL2021376)Show SMILES Nc1nc2c(nc(N)[nH]c2=O)n1[C@@H]1O[C@H](CO)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C10H14N6O5/c11-9-14-6-3(7(20)15-9)13-10(12)16(6)8-5(19)4(18)2(1-17)21-8/h2,4-5,8,17-19H,1H2,(H2,12,13)(H3,11,14,15,20)/t2-,4-,5-,8-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Lyon I
Curated by ChEMBL
| Assay Description
Compound was evaluated for its binding affinity against Toxoplasma gondii purine nucleoside phosphorylase of virulent strain RH (value indicates comp... |
Bioorg Med Chem Lett 12: 977-9 (2002)
BindingDB Entry DOI: 10.7270/Q2HM591J |
More data for this
Ligand-Target Pair | |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50404028

(CHEMBL2021376)Show SMILES Nc1nc2c(nc(N)[nH]c2=O)n1[C@@H]1O[C@H](CO)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C10H14N6O5/c11-9-14-6-3(7(20)15-9)13-10(12)16(6)8-5(19)4(18)2(1-17)21-8/h2,4-5,8,17-19H,1H2,(H2,12,13)(H3,11,14,15,20)/t2-,4-,5-,8-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for inhibition of purine nucleoside phosphorylase using human erythro lysate |
J Med Chem 29: 2034-7 (1986)
BindingDB Entry DOI: 10.7270/Q29P326Z |
More data for this
Ligand-Target Pair | |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50404028

(CHEMBL2021376)Show SMILES Nc1nc2c(nc(N)[nH]c2=O)n1[C@@H]1O[C@H](CO)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C10H14N6O5/c11-9-14-6-3(7(20)15-9)13-10(12)16(6)8-5(19)4(18)2(1-17)21-8/h2,4-5,8,17-19H,1H2,(H2,12,13)(H3,11,14,15,20)/t2-,4-,5-,8-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.33E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for 50% inhibition of purine nucleoside phosphorylase activity by was measured by the conversion of [8-14C]-inosine to [8-14C]... |
J Med Chem 29: 1804-6 (1986)
BindingDB Entry DOI: 10.7270/Q2F76D5J |
More data for this
Ligand-Target Pair | |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50404028

(CHEMBL2021376)Show SMILES Nc1nc2c(nc(N)[nH]c2=O)n1[C@@H]1O[C@H](CO)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C10H14N6O5/c11-9-14-6-3(7(20)15-9)13-10(12)16(6)8-5(19)4(18)2(1-17)21-8/h2,4-5,8,17-19H,1H2,(H2,12,13)(H3,11,14,15,20)/t2-,4-,5-,8-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Defense Medical Center
Curated by ChEMBL
| Assay Description
Compound was evaluated for the inhibitory activity against PNP activity in dialyzed extracts from human erythrocytes |
J Med Chem 36: 1024-31 (1993)
BindingDB Entry DOI: 10.7270/Q2CV4JCQ |
More data for this
Ligand-Target Pair | |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50404028

(CHEMBL2021376)Show SMILES Nc1nc2c(nc(N)[nH]c2=O)n1[C@@H]1O[C@H](CO)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C10H14N6O5/c11-9-14-6-3(7(20)15-9)13-10(12)16(6)8-5(19)4(18)2(1-17)21-8/h2,4-5,8,17-19H,1H2,(H2,12,13)(H3,11,14,15,20)/t2-,4-,5-,8-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Lyon I
Curated by ChEMBL
| Assay Description
Compound was evaluated for its binding affinity against Toxoplasma gondii purine nucleoside phosphorylase from cystic strain ME 49 (value indicates c... |
Bioorg Med Chem Lett 12: 977-9 (2002)
BindingDB Entry DOI: 10.7270/Q2HM591J |
More data for this
Ligand-Target Pair | |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50404028

(CHEMBL2021376)Show SMILES Nc1nc2c(nc(N)[nH]c2=O)n1[C@@H]1O[C@H](CO)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C10H14N6O5/c11-9-14-6-3(7(20)15-9)13-10(12)16(6)8-5(19)4(18)2(1-17)21-8/h2,4-5,8,17-19H,1H2,(H2,12,13)(H3,11,14,15,20)/t2-,4-,5-,8-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Compound was tested for the inhibitory activity against purine nucleoside phosphorylase (PNP) |
J Med Chem 35: 1605-9 (1992)
BindingDB Entry DOI: 10.7270/Q2KW5GPH |
More data for this
Ligand-Target Pair | |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50404028

(CHEMBL2021376)Show SMILES Nc1nc2c(nc(N)[nH]c2=O)n1[C@@H]1O[C@H](CO)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C10H14N6O5/c11-9-14-6-3(7(20)15-9)13-10(12)16(6)8-5(19)4(18)2(1-17)21-8/h2,4-5,8,17-19H,1H2,(H2,12,13)(H3,11,14,15,20)/t2-,4-,5-,8-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.90E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Competitive inhibition of human erythrocyte purine nucleoside phosphorylase assessed as inhibition of guanosine phosphorylysis after 30 mins by Linew... |
J Med Chem 21: 877-82 (1979)
BindingDB Entry DOI: 10.7270/Q2DN46KH |
More data for this
Ligand-Target Pair | |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50404028

(CHEMBL2021376)Show SMILES Nc1nc2c(nc(N)[nH]c2=O)n1[C@@H]1O[C@H](CO)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C10H14N6O5/c11-9-14-6-3(7(20)15-9)13-10(12)16(6)8-5(19)4(18)2(1-17)21-8/h2,4-5,8,17-19H,1H2,(H2,12,13)(H3,11,14,15,20)/t2-,4-,5-,8-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Lyon I
Curated by ChEMBL
| Assay Description
Compound was evaluated for its binding affinity against Toxoplasma gondii purine nucleoside phosphorylase of virulent strain RH (value indicates Non ... |
Bioorg Med Chem Lett 12: 977-9 (2002)
BindingDB Entry DOI: 10.7270/Q2HM591J |
More data for this
Ligand-Target Pair | |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50404028

(CHEMBL2021376)Show SMILES Nc1nc2c(nc(N)[nH]c2=O)n1[C@@H]1O[C@H](CO)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C10H14N6O5/c11-9-14-6-3(7(20)15-9)13-10(12)16(6)8-5(19)4(18)2(1-17)21-8/h2,4-5,8,17-19H,1H2,(H2,12,13)(H3,11,14,15,20)/t2-,4-,5-,8-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibitory activity against Purine nucleoside phosphorylase evaluated by radiochemical assay |
J Med Chem 35: 1451-7 (1992)
BindingDB Entry DOI: 10.7270/Q2MW2HSF |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data