

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50404855 CHEMBL158007
SMILES: CCCCCCCCCCCc1nc2ccccc2[nH]1
InChI Key: InChIKey=GFKNPGTWLJFDKJ-UHFFFAOYSA-N
Data: 4 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cytochrome P450 1A1 (Rattus norvegicus) | BDBM50404855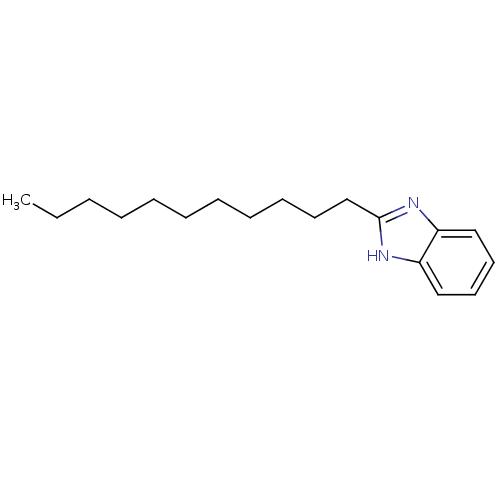 (CHEMBL158007) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.78E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Aryl hydrocarbon hydroxylase in phenobarbitone-treated rats | J Med Chem 25: 622-6 (1982) BindingDB Entry DOI: 10.7270/Q2474C1S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2B1 (Rattus norvegicus) | BDBM50404855 (CHEMBL158007) | PDB UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.51E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory potency to aminopyrine N-demethylase activity (P450) in hepatic microsomes from phenobarbitone-induced rats. | J Med Chem 25: 887-92 (1982) BindingDB Entry DOI: 10.7270/Q2VT1T8J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2B1 (Rattus norvegicus) | BDBM50404855 (CHEMBL158007) | PDB UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Aminopyrine N-demethylase in Phenobarbitone-treated rats | J Med Chem 25: 622-6 (1982) BindingDB Entry DOI: 10.7270/Q2474C1S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A1 (Rattus norvegicus) | BDBM50404855 (CHEMBL158007) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Aryl hydrocarbon hydroxylase in phenobarbitone-treated rats | J Med Chem 25: 622-6 (1982) BindingDB Entry DOI: 10.7270/Q2474C1S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||