

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50405103 CHEMBL2115349
SMILES: CCCCc1ccc(Nc2nc3n(cnc3c(=O)[nH]2)[C@H]2C[C@H](O)[C@@H](CO)O2)cc1
InChI Key: InChIKey=HPXCEMUJBTUFGO-ARFHVFGLSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DNA polymerase (alpha/delta/epsilon) (Homo sapiens (Human)) | BDBM50405103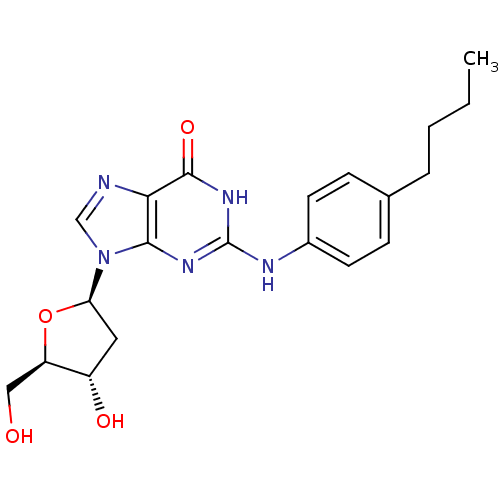 (CHEMBL2115349) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for its inhibitory activity against HeLa DNA polymerase alpha, Ki values were obtained in the absence of dGTP | J Med Chem 27: 175-81 (1984) BindingDB Entry DOI: 10.7270/Q2HH6KMT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase (alpha/delta/epsilon) (Homo sapiens (Human)) | BDBM50405103 (CHEMBL2115349) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for its inhibitory activity against HeLa DNA polymerase alpha, Ki values were obtained in the absence of dGTP | J Med Chem 27: 175-81 (1984) BindingDB Entry DOI: 10.7270/Q2HH6KMT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase (alpha/delta/epsilon) (Homo sapiens (Human)) | BDBM50405103 (CHEMBL2115349) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for its inhibitory activity against HeLa DNA polymerase alpha, Ki values were obtained in the absence of dGTP | J Med Chem 27: 175-81 (1984) BindingDB Entry DOI: 10.7270/Q2HH6KMT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine kinase (Human herpesvirus 1 (strain SC16) (HHV-1) (Human h...) | BDBM50405103 (CHEMBL2115349) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 2.50E+4 | n/a | n/a | n/a | n/a |
Istituto di Genetica Biochimica ed Evoluzionistica Curated by ChEMBL | Assay Description Inhibitory activity against Herpes simplex virus type-I specific thymidine kinase | J Med Chem 31: 1496-500 (1988) BindingDB Entry DOI: 10.7270/Q2930TR7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||