

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50406497 CHEMBL2079606
SMILES: CSCC[C@H](NC(=O)[C@H](Cc1ccc(cc1)-c1nnn[nH]1)NC(C)=O)C(=O)NCC(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CCSC)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O
InChI Key: InChIKey=ILOSQOBMIQVKSA-BGBFCPIGSA-N
Data: 2 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cholecystokinin B receptor (Bos taurus) | BDBM50406497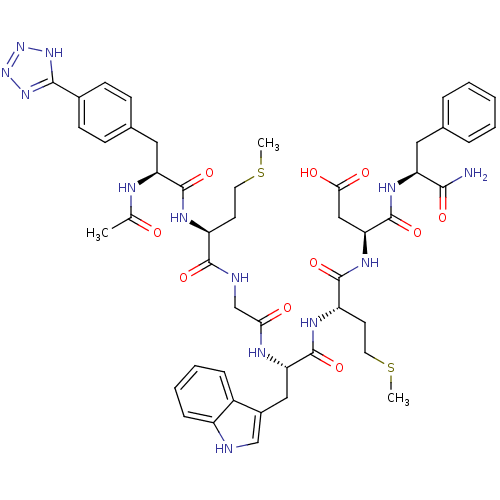 (CHEMBL2079606) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center Curated by ChEMBL | Assay Description Inhibition of [3H]-propanoyl binding to cholecystokinin type B receptor subtype was determined in bovine striatum membranes | J Med Chem 34: 1125-36 (1991) BindingDB Entry DOI: 10.7270/Q27P8XCT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor (RAT) | BDBM50406497 (CHEMBL2079606) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center Curated by ChEMBL | Assay Description Inhibition of [3H]-propanoyl binding to cholecystokinin type A receptor was determined in fresh rat pancreatic tissue membranes | J Med Chem 34: 1125-36 (1991) BindingDB Entry DOI: 10.7270/Q27P8XCT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||