

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50409078 CHEMBL325761
SMILES: Cc1c(C)c(=O)oc2cc(OCc3ccc(F)c(F)c3)ccc12
InChI Key: InChIKey=OAIPPRYRUKGVPD-UHFFFAOYSA-N
Data: 10 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Monoamine Oxidase Type B (MAO-B) (Rattus norvegicus (rat)) | BDBM50409078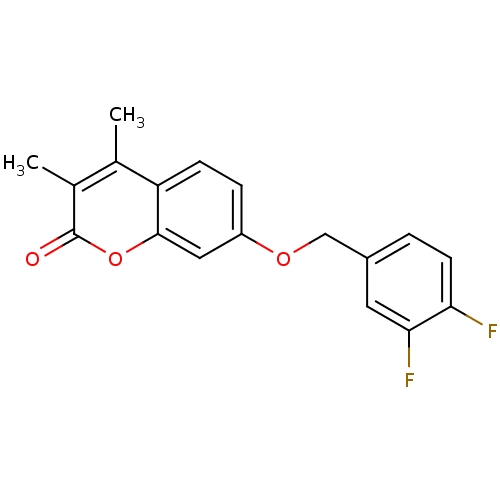 (CHEMBL325761) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Lausanne Curated by ChEMBL | Assay Description Inhibitory activity against monoamine oxidase B | J Med Chem 44: 3195-8 (2001) BindingDB Entry DOI: 10.7270/Q2HQ417K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoamine oxidase (Rattus norvegicus (rat)) | BDBM50409078 (CHEMBL325761) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 123 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Lausanne Curated by ChEMBL | Assay Description Inhibitory activity against monoamine oxidase A | J Med Chem 44: 3195-8 (2001) BindingDB Entry DOI: 10.7270/Q2HQ417K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoamine oxidase (Rattus norvegicus (rat)) | BDBM50409078 (CHEMBL325761) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 123 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Lausanne Curated by ChEMBL | Assay Description Inhibitory effect on monoamine oxidase A, SD on IC50 values < 10% | J Med Chem 43: 4747-58 (2000) BindingDB Entry DOI: 10.7270/Q20K29RT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoamine Oxidase Type B (MAO-B) (Rattus norvegicus (rat)) | BDBM50409078 (CHEMBL325761) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.15 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Lausanne Curated by ChEMBL | Assay Description Inhibitory effect on Monoamine oxidase B, SD on IC50 values < 10% | J Med Chem 43: 4747-58 (2000) BindingDB Entry DOI: 10.7270/Q20K29RT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoamine oxidase (Rattus norvegicus (rat)) | BDBM50409078 (CHEMBL325761) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
North-West University Curated by ChEMBL | Assay Description Competitive inhibition of rat brain MAO-B | Bioorg Med Chem Lett 23: 5498-502 (2013) Article DOI: 10.1016/j.bmcl.2013.08.071 BindingDB Entry DOI: 10.7270/Q26H4MB3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase (flavin-containing) A (Homo sapiens (Human)) | BDBM50409078 (CHEMBL325761) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of recombinant human MAO-A using p-tyramine as substrate assessed as decrease in H2O2 production incubated for 15 mins by fluorimetric met... | Bioorg Med Chem 24: 5929-5940 (2016) Article DOI: 10.1016/j.bmc.2016.09.050 BindingDB Entry DOI: 10.7270/Q2FN1854 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50409078 (CHEMBL325761) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of recombinant human MAO-B using p-tyramine as substrate assessed as decrease in H2O2 production incubated for 15 mins by fluorimetric met... | Bioorg Med Chem 24: 5929-5940 (2016) Article DOI: 10.1016/j.bmc.2016.09.050 BindingDB Entry DOI: 10.7270/Q2FN1854 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoamine Oxidase Type B (MAO-B) (Rattus norvegicus (rat)) | BDBM50409078 (CHEMBL325761) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of rat brain mitochondrial MAOB using kynuramine as substrate assessed as decrease in 4-hydroxyquinoline production by spectrophotometry | Bioorg Med Chem 24: 5929-5940 (2016) Article DOI: 10.1016/j.bmc.2016.09.050 BindingDB Entry DOI: 10.7270/Q2FN1854 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50409078 (CHEMBL325761) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
R.C. Patel Institute of Pharmaceutical Education and Research Curated by ChEMBL | Assay Description Inhibition of MAO-B (unknown origin) | Bioorg Med Chem 21: 2434-50 (2013) Article DOI: 10.1016/j.bmc.2013.02.017 BindingDB Entry DOI: 10.7270/Q25T3PCS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoamine oxidase (Rattus norvegicus (rat)) | BDBM50409078 (CHEMBL325761) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 123 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of rat brain mitochondrial MAOA using kynuramine as substrate assessed as decrease in 4-hydroxyquinoline production by spectrophotometry | Bioorg Med Chem 24: 5929-5940 (2016) Article DOI: 10.1016/j.bmc.2016.09.050 BindingDB Entry DOI: 10.7270/Q2FN1854 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||