

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50409527 CHEMBL2112283
SMILES: CN(C)CCCN(C)CCNC(=O)[C@@H]1CCCN1S(=O)(=O)c1ccc(NNC(=O)NC2c3ccccc3CCc3ccccc23)c(c1)[N+]([O-])=O
InChI Key: InChIKey=NSKPGTTUWOXHBF-HKBQPEDESA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BDKRB2 (Homo sapiens (Human)) | BDBM50409527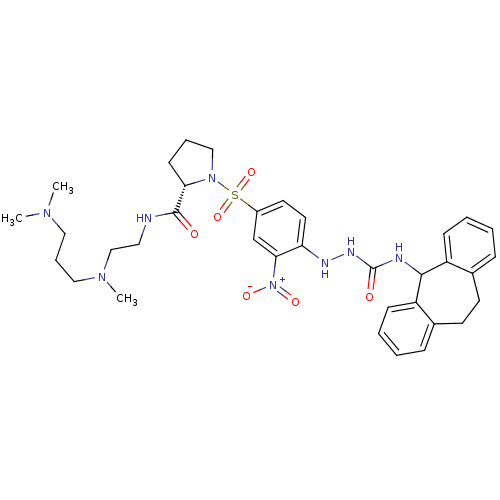 (CHEMBL2112283) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.790 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Medical Sciences Curated by ChEMBL | Assay Description Displacement of [3H]BK (1 nM) from human Bradykinin receptor B2 expressed in Cos-7 cells | J Med Chem 45: 2160-72 (2002) BindingDB Entry DOI: 10.7270/Q2X067SG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| BDKRB2 (RAT) | BDBM50409527 (CHEMBL2112283) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 10.7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Medical Sciences Curated by ChEMBL | Assay Description Inhibition of specific binding of [3H]-BK (1 nM) to rat Bradykinin receptor B2 by 50% in NG108-15 cell membranes | J Med Chem 45: 2160-72 (2002) BindingDB Entry DOI: 10.7270/Q2X067SG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| BDKRB2 (Homo sapiens (Human)) | BDBM50409527 (CHEMBL2112283) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Medical Sciences Curated by ChEMBL | Assay Description Antagonism of the [Ca2+] efflux actions of human Bradykinin receptor B2 (WI38 fibroblasts) | J Med Chem 45: 2160-72 (2002) BindingDB Entry DOI: 10.7270/Q2X067SG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| BDKRB2 (Homo sapiens (Human)) | BDBM50409527 (CHEMBL2112283) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 91 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Medical Sciences Curated by ChEMBL | Assay Description Antagonism of the [Ca2+] efflux actions of human Bradykinin receptor B2 (SK-N-SH neuroblastoma) | J Med Chem 45: 2160-72 (2002) BindingDB Entry DOI: 10.7270/Q2X067SG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| BDKRB2 (RAT) | BDBM50409527 (CHEMBL2112283) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Medical Sciences Curated by ChEMBL | Assay Description Inhibition of Bradykinin receptor B2-mediated contractions of rat uterus smooth muscle | J Med Chem 45: 2160-72 (2002) BindingDB Entry DOI: 10.7270/Q2X067SG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| BDKRB2 (RAT) | BDBM50409527 (CHEMBL2112283) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 105 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Medical Sciences Curated by ChEMBL | Assay Description Inhibition of increase in [Ca2+] efflux from NG108-15 cells caused by activation of rat Bradykinin receptor B2 | J Med Chem 45: 2160-72 (2002) BindingDB Entry DOI: 10.7270/Q2X067SG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||