

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50415097 CHEMBL570897
SMILES: [O-][N+](=O)c1ccc(N2CCCC2)c2ccccc12
InChI Key: InChIKey=FLUWUUPEIABQQO-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cathepsin B (Homo sapiens (Human)) | BDBM50415097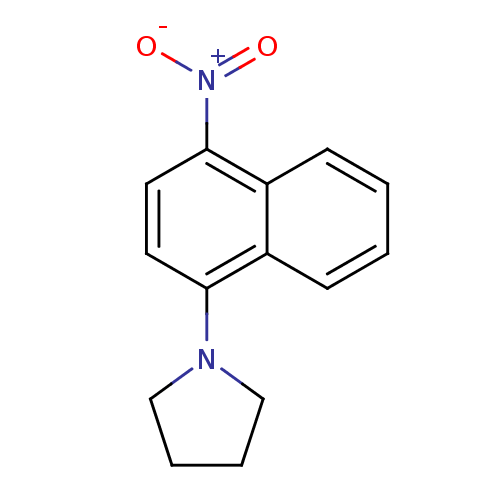 (CHEMBL570897) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | 3.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ljubljana Curated by ChEMBL | Assay Description Noncompetitive inhibition of human recombinant cathepsin B exopeptidase activity using Abz-Gly-Ile-Val-Arg-Ala-Lys(Dnp)-OH substrate | J Med Chem 56: 521-33 (2013) Article DOI: 10.1021/jm301544x BindingDB Entry DOI: 10.7270/Q24Q7W9J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin B (Homo sapiens (Human)) | BDBM50415097 (CHEMBL570897) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | 1.70E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ljubljana Curated by ChEMBL | Assay Description Mixed inhibition of human recombinant cathepsin B endopeptidase activity using Z-Arg-Arg-AMC substrate assessed as inhibition constant for enzyme-inh... | J Med Chem 56: 521-33 (2013) Article DOI: 10.1021/jm301544x BindingDB Entry DOI: 10.7270/Q24Q7W9J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM50415097 (CHEMBL570897) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | 2.45E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ljubljana Curated by ChEMBL | Assay Description Competitive inhibition of human recombinant cathepsin L endopeptidase activity using Z-FR-AMC substrate expressed in Escherichia coli assessed as inh... | J Med Chem 56: 521-33 (2013) Article DOI: 10.1021/jm301544x BindingDB Entry DOI: 10.7270/Q24Q7W9J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pro-cathepsin H (Homo sapiens (Human)) | BDBM50415097 (CHEMBL570897) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | 2.51E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ljubljana Curated by ChEMBL | Assay Description Uncompetitive inhibition of human liver cathepsin H endopeptidase activity using R-AMC substrate assessed as inhibition constant for enzyme-substrate... | J Med Chem 56: 521-33 (2013) Article DOI: 10.1021/jm301544x BindingDB Entry DOI: 10.7270/Q24Q7W9J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin B (Homo sapiens (Human)) | BDBM50415097 (CHEMBL570897) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | 2.77E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ljubljana Curated by ChEMBL | Assay Description Mixed inhibition of human recombinant cathepsin B endopeptidase activity using Z-Arg-Arg-AMC substrate assessed as inhibition constant for enzyme-sub... | J Med Chem 56: 521-33 (2013) Article DOI: 10.1021/jm301544x BindingDB Entry DOI: 10.7270/Q24Q7W9J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen Receptor (Mus musculus) | BDBM50415097 (CHEMBL570897) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 6.31 | n/a | n/a | n/a | n/a |
ACADIA Pharmaceuticals AB Curated by ChEMBL | Assay Description Agonist activity at androgen receptor in mouse NIH3T3 cells transiently transfected with beta-galactosidase reporter gene assessed as cellular transf... | J Med Chem 52: 7186-91 (2009) Article DOI: 10.1021/jm901149c BindingDB Entry DOI: 10.7270/Q2SJ1MV9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen Receptor (Homo sapiens (Human)) | BDBM50415097 (CHEMBL570897) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd Curated by ChEMBL | Assay Description Displacement of [3H]mibolerone from androgen receptor (unknown origin) after 3 hrs | Bioorg Med Chem 23: 2568-78 (2015) Article DOI: 10.1016/j.bmc.2015.03.032 BindingDB Entry DOI: 10.7270/Q2JM2C94 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen Receptor (Homo sapiens (Human)) | BDBM50415097 (CHEMBL570897) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 41 | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd Curated by ChEMBL | Assay Description Modulation of androgen receptor (unknown origin) expressed in Cos7 cells assessed as luciferase activity after 24 hrs | Bioorg Med Chem 23: 2568-78 (2015) Article DOI: 10.1016/j.bmc.2015.03.032 BindingDB Entry DOI: 10.7270/Q2JM2C94 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||