

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
null
SMILES: Fc1cnc(nc1)N1CCN(Cc2cnc([nH]2)-c2ccccc2)CC1
InChI Key: InChIKey=GKLMXCIEUYGUNY-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50418100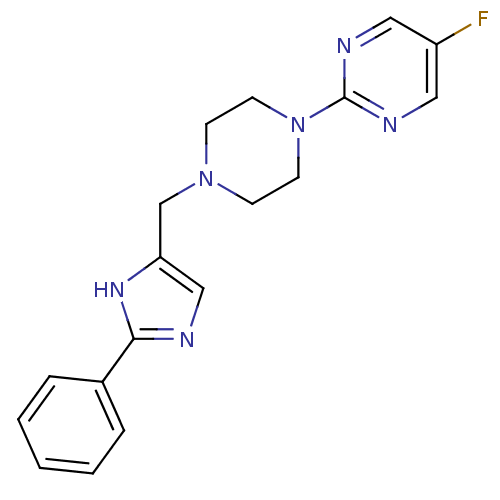 (CHEMBL1743355 | SCH-66712) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kalamazoo College Curated by ChEMBL | Assay Description Inhibition of CYP2D6 (unknown origin) | Drug Metab Dispos 39: 974-83 (2011) Article DOI: 10.1124/dmd.110.037630 BindingDB Entry DOI: 10.7270/Q22N541S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50418100 (CHEMBL1743355 | SCH-66712) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Mechanism based inhibition of human cytochrome P450 2D6 measured by dextromethorphan O-demethylation using recombinant CYP2D6 | Curr Drug Metab 6: 413-54 (2005) BindingDB Entry DOI: 10.7270/Q2VQ33X3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||