

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50420186 ZOSUQUIDAR::ZOSUQUIDAR TRIHYDROCHLORIDE
SMILES: O[C@@H](COc1cccc2ncccc12)CN1CCN(CC1)[C@@H]1c2ccccc2[C@@H]2[C@H](c3ccccc13)C2(F)F
InChI Key: InChIKey=IHOVFYSQUDPMCN-DBEBIPAYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P-glycoprotein 1 (Homo sapiens (Human)) | BDBM50420186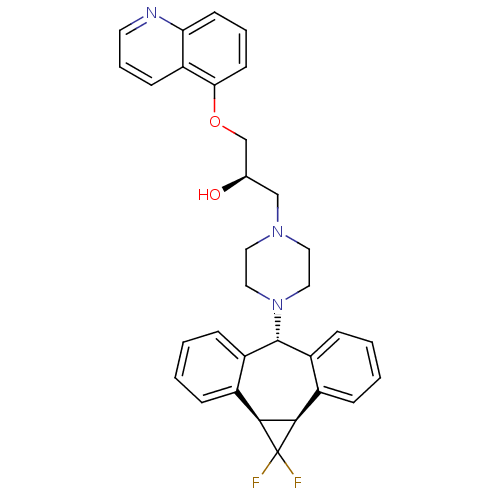 (ZOSUQUIDAR | ZOSUQUIDAR TRIHYDROCHLORIDE) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | PubMed | n/a | n/a | n/a | 73 | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description TP_TRANSPORTER: binding in membrane vesicle from CEM/VLB100 cells | J Pharmacol Exp Ther 290: 854-62 (1999) BindingDB Entry DOI: 10.7270/Q2G1623R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P-glycoprotein 1 (Homo sapiens (Human)) | BDBM50420186 (ZOSUQUIDAR | ZOSUQUIDAR TRIHYDROCHLORIDE) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University School of Medicine Curated by ChEMBL | Assay Description TP_TRANSPORTER: inhibition of Digoxin transepithelial transport (basal to apical) (Digoxin: 5 uM) in Caco-2 cells | Drug Metab Dispos 28: 655-60 (2000) BindingDB Entry DOI: 10.7270/Q2959JTX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P-glycoprotein 1 (Homo sapiens (Human)) | BDBM50420186 (ZOSUQUIDAR | ZOSUQUIDAR TRIHYDROCHLORIDE) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University School of Medicine Curated by ChEMBL | Assay Description TP_TRANSPORTER: inhibition of Nelfinavir transepithelial transport (basal to apical) (Nelfinavir: 0.02 uM) in Caco-2 cells | Drug Metab Dispos 28: 655-60 (2000) BindingDB Entry DOI: 10.7270/Q2959JTX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P-glycoprotein 1 (Homo sapiens (Human)) | BDBM50420186 (ZOSUQUIDAR | ZOSUQUIDAR TRIHYDROCHLORIDE) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University School of Medicine Curated by ChEMBL | Assay Description TP_TRANSPORTER: inhibition of Indinavir transepithelial transport (basal to apical) (Indinavir: 0.05 uM) in Caco-2 cells | Drug Metab Dispos 28: 655-60 (2000) BindingDB Entry DOI: 10.7270/Q2959JTX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Multidrug resistance protein 1/Multidrug resistance associated protein 1 (Homo sapiens (Human)) | BDBM50420186 (ZOSUQUIDAR | ZOSUQUIDAR TRIHYDROCHLORIDE) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.49E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Key Laboratory of Molecular Pharmacology and Drug Evaluation (Yantai University) Curated by ChEMBL | Assay Description Reversal of P-gp-mediated drug resistance in human KBV cells assessed as potentiation of paclitaxel-induced cytotoxicity by measuring paclitaxel IC50... | Eur J Med Chem 161: 364-377 (2019) Article DOI: 10.1016/j.ejmech.2018.10.033 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Multidrug resistance protein 1/Multidrug resistance associated protein 1 (Homo sapiens (Human)) | BDBM50420186 (ZOSUQUIDAR | ZOSUQUIDAR TRIHYDROCHLORIDE) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 59 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Vienna Curated by ChEMBL | Assay Description Inhibition of P-glycoprotein-mediated daunorubicin efflux from human CCRF-CEM/VCR1000 cells after 240 secs by FACS flow cytometric analysis | J Med Chem 55: 3261-73 (2012) Article DOI: 10.1021/jm201705f BindingDB Entry DOI: 10.7270/Q2ZP48ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Multidrug resistance protein 1/Multidrug resistance associated protein 1 (Homo sapiens (Human)) | BDBM50420186 (ZOSUQUIDAR | ZOSUQUIDAR TRIHYDROCHLORIDE) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Heidelberg Curated by ChEMBL | Assay Description TP_TRANSPORTER: cell accumulation of calcein in L-MDR1 cells | J Pharmacol Exp Ther 305: 197-204 (2003) Article DOI: 10.1124/jpet.102.046532 BindingDB Entry DOI: 10.7270/Q2445QCR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Multidrug resistance protein 1/Multidrug resistance associated protein 1 (Homo sapiens (Human)) | BDBM50420186 (ZOSUQUIDAR | ZOSUQUIDAR TRIHYDROCHLORIDE) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.23E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Key Laboratory of Molecular Pharmacology and Drug Evaluation (Yantai University) Curated by ChEMBL | Assay Description Reversal of P-gp-mediated drug resistance in human KBV cells assessed as potentiation of paclitaxel-induced cytotoxicity by measuring paclitaxel IC50... | Eur J Med Chem 161: 364-377 (2019) Article DOI: 10.1016/j.ejmech.2018.10.033 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P-glycoprotein 1 (Homo sapiens (Human)) | BDBM50420186 (ZOSUQUIDAR | ZOSUQUIDAR TRIHYDROCHLORIDE) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | PubMed | n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University School of Medicine Curated by ChEMBL | Assay Description TP_TRANSPORTER: inhibition of Indinavir transepithelial transport (basal to apical) (Indinavir: 0.05 uM) in Caco-2 cells | Drug Metab Dispos 28: 655-60 (2000) BindingDB Entry DOI: 10.7270/Q2959JTX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||