

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50422013 LOBELINE::Lobeline Hydrochloride
SMILES: CN1[C@@H](C[C@H](O)c2ccccc2)CCC[C@H]1CC(=O)c1ccccc1
InChI Key: InChIKey=MXYUKLILVYORSK-HKBOAZHASA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Neuronal acetylcholine receptor Alpha-4/Beta-2 (Homo sapiens (Human)) | BDBM50422013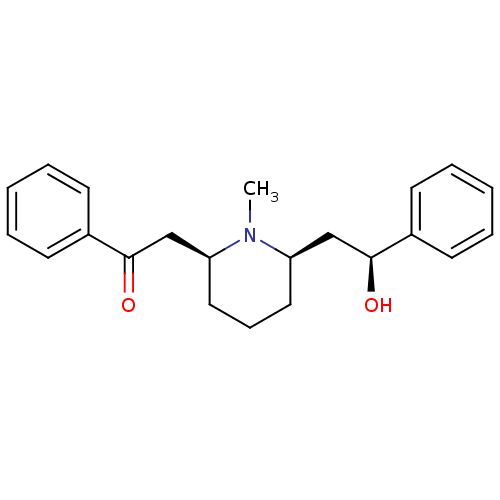 (LOBELINE | Lobeline Hydrochloride) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Hawai'i at Hilo Curated by ChEMBL | Assay Description Binding affinity to alpha3beta4 nicotine acetylcholine receptor (unknown origin) after 90 mins by radioligand displacement assay | Bioorg Med Chem 23: 4375-89 (2015) Article DOI: 10.1016/j.bmc.2015.06.034 BindingDB Entry DOI: 10.7270/Q2C24Z6H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor Alpha-4/Beta-2 (Homo sapiens (Human)) | BDBM50422013 (LOBELINE | Lobeline Hydrochloride) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Copenhagen Curated by ChEMBL | Assay Description Binding affinity to alpha4beta2 (unknown origin) | Eur J Med Chem 102: 425-44 (2015) BindingDB Entry DOI: 10.7270/Q2765H4R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor Alpha-4/Beta-2 (Homo sapiens (Human)) | BDBM50422013 (LOBELINE | Lobeline Hydrochloride) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Copenhagen Curated by ChEMBL | Assay Description Binding affinity to alpha4beta2 (unknown origin) | Eur J Med Chem 102: 425-44 (2015) BindingDB Entry DOI: 10.7270/Q2765H4R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor (Rattus norvegicus (Rat)) | BDBM50422013 (LOBELINE | Lobeline Hydrochloride) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 66 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Hawai'i at Hilo Curated by ChEMBL | Assay Description Binding affinity to alpha3beta4 nicotine acetylcholine receptor (unknown origin) after 90 mins by radioligand displacement assay | Bioorg Med Chem 23: 4375-89 (2015) Article DOI: 10.1016/j.bmc.2015.06.034 BindingDB Entry DOI: 10.7270/Q2C24Z6H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50422013 (LOBELINE | Lobeline Hydrochloride) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | PubMed | 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut f£r Toxikologie Curated by ChEMBL | Assay Description Inhibitory constant for cytochrome P450 2D6 | J Med Chem 36: 1136-45 (1993) BindingDB Entry DOI: 10.7270/Q2GM87X6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vesicular monoamine transporter 2 (VMAT2) (Rattus norvegicus (Rat)) | BDBM50422013 (LOBELINE | Lobeline Hydrochloride) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kentucky Curated by ChEMBL | Assay Description Inhibition of [3H]dopamine uptake at VMAT2 in rat brain synaptic vesicle by liquid scintillation spectroscopy | J Med Chem 52: 7878-82 (2009) Article DOI: 10.1021/jm900770h BindingDB Entry DOI: 10.7270/Q25X29V5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vesicular monoamine transporter 2 (VMAT2) (Rattus norvegicus (Rat)) | BDBM50422013 (LOBELINE | Lobeline Hydrochloride) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 2.76E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kentucky Curated by ChEMBL | Assay Description Displacement of [3H]DTBZ from VMAT2 dihydrotetrabenazine binding site in rat brain synaptic vesicle by scintillation counting | J Med Chem 52: 7878-82 (2009) Article DOI: 10.1021/jm900770h BindingDB Entry DOI: 10.7270/Q25X29V5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Synaptic vesicular amine transporter (Homo sapiens (Human)) | BDBM50422013 (LOBELINE | Lobeline Hydrochloride) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 2.76E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kentucky Curated by ChEMBL | Assay Description Binding affinity to VMAT2 receptor | Bioorg Med Chem 18: 640-9 (2010) Article DOI: 10.1016/j.bmc.2009.12.002 BindingDB Entry DOI: 10.7270/Q2XS5WB2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vesicular monoamine transporter 2 (VMAT2) (Rattus norvegicus (Rat)) | BDBM50422013 (LOBELINE | Lobeline Hydrochloride) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 2.76E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kentucky Curated by ChEMBL | Assay Description Inhibition of [3H]DTBZ binding to VMAT2 in rat synaptic vesicle membrane | Bioorg Med Chem Lett 16: 5018-21 (2006) Article DOI: 10.1016/j.bmcl.2006.07.070 BindingDB Entry DOI: 10.7270/Q2513XTK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vesicular monoamine transporter 2 (VMAT2) (Rattus norvegicus (Rat)) | BDBM50422013 (LOBELINE | Lobeline Hydrochloride) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 5.46E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kentucky Curated by ChEMBL | Assay Description Displacement of [3H]MTBZ from VMAT2 in rat whole brain vesicles by liquid scintillation spectrophotometry | Bioorg Med Chem 18: 640-9 (2010) Article DOI: 10.1016/j.bmc.2009.12.002 BindingDB Entry DOI: 10.7270/Q2XS5WB2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (RAT) | BDBM50422013 (LOBELINE | Lobeline Hydrochloride) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.12E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford Curated by ChEMBL | Assay Description Displacement of [3H]mepyramine from histamine H1 receptor in Sprague-Dawley rat brain membrane after 2 hr by scintillation counting | J Med Chem 55: 7054-60 (2012) Article DOI: 10.1021/jm300671m BindingDB Entry DOI: 10.7270/Q2FN17BZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholine Binding protein (Lymnaea stagnalis) | BDBM50422013 (LOBELINE | Lobeline Hydrochloride) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | 631 | n/a | n/a | n/a | n/a | n/a |
University of Copenhagen Curated by ChEMBL | Assay Description Binding affinity to Lymnaea stagnalis AChBP after 300 seconds by SPR biosensor analysis | Eur J Med Chem 102: 425-44 (2015) BindingDB Entry DOI: 10.7270/Q2765H4R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholine Binding protein (Lymnaea stagnalis) | BDBM50422013 (LOBELINE | Lobeline Hydrochloride) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | 630 | n/a | n/a | n/a | n/a | n/a |
University of Copenhagen Curated by ChEMBL | Assay Description Binding affinity to Lymnaea stagnalis AChBP after 300 seconds by SPR biosensor analysis | Eur J Med Chem 102: 425-44 (2015) BindingDB Entry DOI: 10.7270/Q2765H4R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50422013 (LOBELINE | Lobeline Hydrochloride) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.47E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford Curated by ChEMBL | Assay Description Antagonist activity at H1 receptor in human HeLa cells assessed as inhibition of histamine-induced Ca2+ release by using fura-2AM-based fluorescence ... | J Med Chem 55: 7054-60 (2012) Article DOI: 10.1021/jm300671m BindingDB Entry DOI: 10.7270/Q2FN17BZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||