

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50422131 CHEMBL2303887
SMILES: OC[C@H]1O[C@H](Oc2ccccc2-c2cccc(CC(O)=O)c2)[C@@H](O)[C@H](O)[C@@H]1O
InChI Key: InChIKey=NCYFXLIEKHHURF-XLSIIYIISA-N
Data: 6 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Leukocyte adhesion molecule-1 (Mus musculus) | BDBM50422131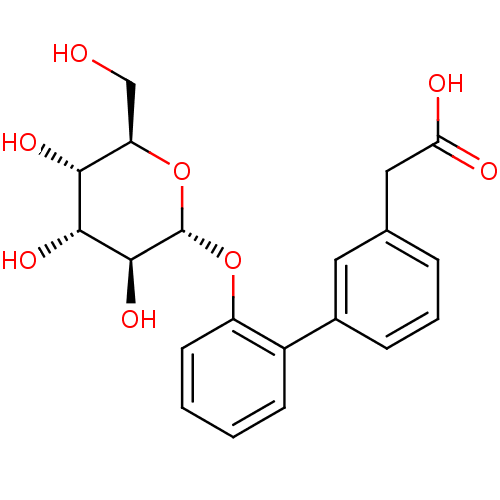 (CHEMBL2303887) | Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Texas Biotechnology Corporation Curated by ChEMBL | Assay Description In vitro inhibitory activity against sialyl Lewis X expressing HL60 cell binding Selectin L | J Med Chem 38: 4976-84 (1996) BindingDB Entry DOI: 10.7270/Q2B56KCP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P-selectin/P-selectin glycoprotein ligand 1 (Homo sapiens (Human)) | BDBM50422131 (CHEMBL2303887) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.60E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Texas Biotechnology Corporation Curated by ChEMBL | Assay Description In vitro inhibitory activity against sialyl Lewis X expressing HL60 cell binding Selectin P | J Med Chem 38: 4976-84 (1996) BindingDB Entry DOI: 10.7270/Q2B56KCP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Selectin E (Homo sapiens (Human)) | BDBM50422131 (CHEMBL2303887) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.10E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Texas Biotechnology Corporation Curated by ChEMBL | Assay Description Tested in vitro for the concentration to inhibit sLex bearing HL-60 cells binding to Selectin E-IgG fusion proteins by 50%. | J Med Chem 41: 1099-111 (1998) Article DOI: 10.1021/jm9704917 BindingDB Entry DOI: 10.7270/Q2BP03G4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P-selectin/P-selectin glycoprotein ligand 1 (Homo sapiens (Human)) | BDBM50422131 (CHEMBL2303887) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.60E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Texas Biotechnology Corporation Curated by ChEMBL | Assay Description Tested in vitro for the concentration to inhibit sLex bearing HL-60 cells binding to Selectin P-IgG fusion proteins | J Med Chem 41: 1099-111 (1998) Article DOI: 10.1021/jm9704917 BindingDB Entry DOI: 10.7270/Q2BP03G4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukocyte adhesion molecule-1 (Homo sapiens (Human)) | BDBM50422131 (CHEMBL2303887) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.10E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Texas Biotechnology Corporation Curated by ChEMBL | Assay Description Tested in vitro for the concentration to inhibit sLex bearing HL-60 cells binding to Selectin L-IgG fusion proteins | J Med Chem 41: 1099-111 (1998) Article DOI: 10.1021/jm9704917 BindingDB Entry DOI: 10.7270/Q2BP03G4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Selectin E (Homo sapiens (Human)) | BDBM50422131 (CHEMBL2303887) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Texas Biotechnology Corporation Curated by ChEMBL | Assay Description In vitro inhibition of HL60 (human leukemia) cell binding to human recombinant e-selectin. | J Med Chem 38: 4976-84 (1996) BindingDB Entry DOI: 10.7270/Q2B56KCP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||