

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50423541 DANAZOL::Danocrine::WIN-17757
SMILES: C[C@]12CC[C@H]3[C@@H](CCC4=Cc5oncc5C[C@]34C)[C@@H]1CC[C@@]2(O)C#C
InChI Key: InChIKey=POZRVZJJTULAOH-LHZXLZLDSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cytochrome P450 2J2 (Homo sapiens (Human)) | BDBM50423541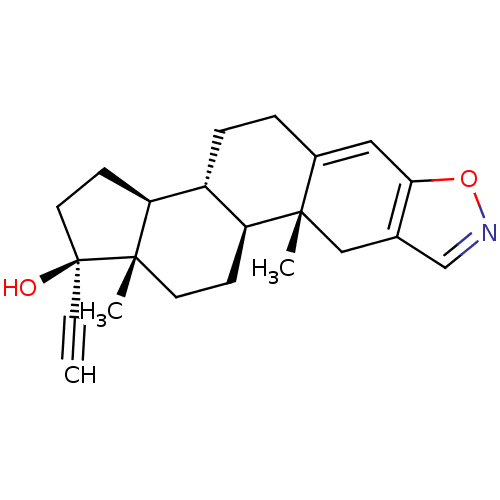 (DANAZOL | Danocrine | WIN-17757) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem Similars | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Mixed inhibition of recombinant CYP2J2 (unknown origin)-mediated astemizole O-demethylation in presence of NADPH | Drug Metab Dispos 40: 943-51 (2012) Article DOI: 10.1124/dmd.111.043505 BindingDB Entry DOI: 10.7270/Q2PN97D2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50423541 (DANAZOL | Danocrine | WIN-17757) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 1.44E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of CYP2C9 in human liver microsomes using diclofenac as substrate after 8 mins by LC-MS/MS analysis | Drug Metab Dispos 40: 943-51 (2012) Article DOI: 10.1124/dmd.111.043505 BindingDB Entry DOI: 10.7270/Q2PN97D2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2J2 (Homo sapiens (Human)) | BDBM50423541 (DANAZOL | Danocrine | WIN-17757) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 77 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of recombinant CYP2J2 (unknown origin)-mediated terfenadine hydroxylation in presence of NADPH | Drug Metab Dispos 40: 943-51 (2012) Article DOI: 10.1124/dmd.111.043505 BindingDB Entry DOI: 10.7270/Q2PN97D2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2J2 (Homo sapiens (Human)) | BDBM50423541 (DANAZOL | Danocrine | WIN-17757) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of recombinant CYP2J2 (unknown origin)-mediated astemizole O-demethylation in presence of NADPH | Drug Metab Dispos 40: 943-51 (2012) Article DOI: 10.1124/dmd.111.043505 BindingDB Entry DOI: 10.7270/Q2PN97D2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C8 (Homo sapiens (Human)) | BDBM50423541 (DANAZOL | Danocrine | WIN-17757) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 1.95E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of CYP2C8 in human liver microsomes using paclitaxel as substrate after 8 mins by LC-MS/MS analysis | Drug Metab Dispos 40: 943-51 (2012) Article DOI: 10.1124/dmd.111.043505 BindingDB Entry DOI: 10.7270/Q2PN97D2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2J2 (Homo sapiens (Human)) | BDBM50423541 (DANAZOL | Danocrine | WIN-17757) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of CYP2J2 in human liver microsomes using 7 probe cocktail containing phenacetin, paclitaxel, diclofenac, S-mephenytoin, dextromethorphan,... | Drug Metab Dispos 40: 943-51 (2012) Article DOI: 10.1124/dmd.111.043505 BindingDB Entry DOI: 10.7270/Q2PN97D2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bile salt export pump (Homo sapiens (Human)) | BDBM50423541 (DANAZOL | Danocrine | WIN-17757) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | <1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human BSEP expressed in baculovirus transfected fall armyworm Sf21 cell membranes vesicles assessed as reduction in ATP-dependent [3H]-... | Drug Metab Dispos 40: 2332-41 (2012) Article DOI: 10.1124/dmd.112.047068 BindingDB Entry DOI: 10.7270/Q2ZP488M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2J2 (Homo sapiens (Human)) | BDBM50423541 (DANAZOL | Danocrine | WIN-17757) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of recombinant CYP2J2 (unknown origin)-mediated astemizole O-demethylation preincubated for 30 mins followed by substrate addition in pres... | Drug Metab Dispos 40: 943-51 (2012) Article DOI: 10.1124/dmd.111.043505 BindingDB Entry DOI: 10.7270/Q2PN97D2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50423541 (DANAZOL | Danocrine | WIN-17757) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem Similars | DrugBank Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of CYP3A4 in human liver microsomes using midazolam as substrate after 8 mins by LC-MS/MS analysis | Drug Metab Dispos 40: 943-51 (2012) Article DOI: 10.1124/dmd.111.043505 BindingDB Entry DOI: 10.7270/Q2PN97D2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C19 (Homo sapiens (Human)) | BDBM50423541 (DANAZOL | Danocrine | WIN-17757) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of CYP2C19 in human liver microsomes using S-mephenytoin as substrate after 8 mins by LC-MS/MS analysis | Drug Metab Dispos 40: 943-51 (2012) Article DOI: 10.1124/dmd.111.043505 BindingDB Entry DOI: 10.7270/Q2PN97D2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A (Homo sapiens (Human)) | BDBM50423541 (DANAZOL | Danocrine | WIN-17757) | PDB MMDB Reactome pathway KEGG B.MOAD DrugBank GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of CYP1A2 in human liver microsomes using phenacetin as substrate after 8 mins by LC-MS/MS analysis | Drug Metab Dispos 40: 943-51 (2012) Article DOI: 10.1124/dmd.111.043505 BindingDB Entry DOI: 10.7270/Q2PN97D2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Testis-specific androgen-binding protein (Homo sapiens (Human)) | BDBM50423541 (DANAZOL | Danocrine | WIN-17757) | PDB MMDB NCI pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem Similars | DrugBank PDB Article PubMed | n/a | n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a |
University of British Columbia Curated by ChEMBL | Assay Description Displacement of [3H]5alpha dihydrotestosterone from human sex hormone binding globulin | J Med Chem 51: 2047-56 (2008) Article DOI: 10.1021/jm7011485 BindingDB Entry DOI: 10.7270/Q2RX9DC2 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50423541 (DANAZOL | Danocrine | WIN-17757) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 2.74E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of CYP2D6 in human liver microsomes using dextromethorphan as substrate after 8 mins by LC-MS/MS analysis | Drug Metab Dispos 40: 943-51 (2012) Article DOI: 10.1124/dmd.111.043505 BindingDB Entry DOI: 10.7270/Q2PN97D2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2J2 (Homo sapiens (Human)) | BDBM50423541 (DANAZOL | Danocrine | WIN-17757) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of CYP2J2-mediated astemizole O-demethylation in human liver microsomes after 8 mins by LC-MS/MS analysis | Drug Metab Dispos 40: 943-51 (2012) Article DOI: 10.1124/dmd.111.043505 BindingDB Entry DOI: 10.7270/Q2PN97D2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||