

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50424075 (-)-Corydalmine::CHEMBL448891
SMILES: COc1cc2CCN3Cc4c(C[C@H]3c2cc1OC)ccc(O)c4OC
InChI Key: InChIKey=DIHXHTWYVOYYDC-INIZCTEOSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM50424075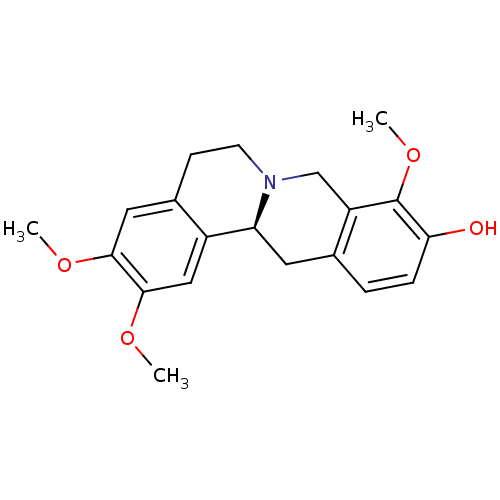 ((-)-Corydalmine | CHEMBL448891) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Displacement of [3H]SCH23390 from dopamine D1 receptor (unknown origin) expressed in human HEK293 cells by liquid scintillation counter | Bioorg Med Chem 21: 856-68 (2013) Article DOI: 10.1016/j.bmc.2012.12.016 BindingDB Entry DOI: 10.7270/Q2JD4Z4M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM50424075 ((-)-Corydalmine | CHEMBL448891) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | 107 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Displacement of [3H]SCH23390 from human dopamine D1 receptor after 1 hr | Bioorg Med Chem Lett 27: 1437-1440 (2017) BindingDB Entry DOI: 10.7270/Q24X5B1N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50424075 ((-)-Corydalmine | CHEMBL448891) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 149 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from 5-HT1A receptor (unknown origin) expressed in HEK293 cells by liquid scintillation counter | Bioorg Med Chem 21: 856-68 (2013) Article DOI: 10.1016/j.bmc.2012.12.016 BindingDB Entry DOI: 10.7270/Q2JD4Z4M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1B) dopamine receptor (Homo sapiens (Human)) | BDBM50424075 ((-)-Corydalmine | CHEMBL448891) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | 242 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Displacement of [3H]SCH23390 from human dopamine D5 receptor after 1 hr | Bioorg Med Chem Lett 27: 1437-1440 (2017) BindingDB Entry DOI: 10.7270/Q24X5B1N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50424075 ((-)-Corydalmine | CHEMBL448891) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 305 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Displacement of [3H]spiperone from dopamine D2 receptor (unknown origin) expressed in human HEK293 cells by liquid scintillation counter | Bioorg Med Chem 21: 856-68 (2013) Article DOI: 10.1016/j.bmc.2012.12.016 BindingDB Entry DOI: 10.7270/Q2JD4Z4M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50424075 ((-)-Corydalmine | CHEMBL448891) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | 533 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Displacement of [3H]N-methylspiperone from human dopamine D2 receptor after 1 hr | Bioorg Med Chem Lett 27: 1437-1440 (2017) BindingDB Entry DOI: 10.7270/Q24X5B1N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(4) dopamine receptor (Homo sapiens (Human)) | BDBM50424075 ((-)-Corydalmine | CHEMBL448891) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Displacement of [3H]N-methylspiperone from human dopamine D4 receptor after 1 hr | Bioorg Med Chem Lett 27: 1437-1440 (2017) BindingDB Entry DOI: 10.7270/Q24X5B1N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50424075 ((-)-Corydalmine | CHEMBL448891) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | 1.71E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Displacement of [3H]N-methylspiperone from human dopamine D3 receptor after 1 hr | Bioorg Med Chem Lett 27: 1437-1440 (2017) BindingDB Entry DOI: 10.7270/Q24X5B1N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50424075 ((-)-Corydalmine | CHEMBL448891) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sunchon National University Curated by ChEMBL | Assay Description Inhibition of recombinant human MAO-B using benzylamine as substrate incubated for 30 mins by spectrophotometric method | Bioorg Med Chem Lett 28: 2403-2407 (2018) Article DOI: 10.1016/j.bmcl.2018.06.023 BindingDB Entry DOI: 10.7270/Q23R0WCT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase (flavin-containing) A (Homo sapiens (Human)) | BDBM50424075 ((-)-Corydalmine | CHEMBL448891) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sunchon National University Curated by ChEMBL | Assay Description Inhibition of recombinant human MAO-A using kynuramine as substrate incubated for 20 mins by spectrophotometric method | Bioorg Med Chem Lett 28: 2403-2407 (2018) Article DOI: 10.1016/j.bmcl.2018.06.023 BindingDB Entry DOI: 10.7270/Q23R0WCT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM50424075 ((-)-Corydalmine | CHEMBL448891) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.35E+3 | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Agonist activity at dopamine D1 receptor (unknown origin) expressed in HEK293 cells assessed as stimulation of [35S]GTPgammaS binding by scintillatio... | Bioorg Med Chem 21: 856-68 (2013) Article DOI: 10.1016/j.bmc.2012.12.016 BindingDB Entry DOI: 10.7270/Q2JD4Z4M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM50424075 ((-)-Corydalmine | CHEMBL448891) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.13E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Antagonist activity at dopamine D1 receptor (unknown origin) expressed in HEK293 cells assessed as inhibition of [35S]GTPgammaS binding by scintillat... | Bioorg Med Chem 21: 856-68 (2013) Article DOI: 10.1016/j.bmc.2012.12.016 BindingDB Entry DOI: 10.7270/Q2JD4Z4M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50424075 ((-)-Corydalmine | CHEMBL448891) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.87E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Antagonist activity at dopamine D2 receptor (unknown origin) expressed in HEK293 cells assessed as inhibition of [35S]GTPgammaS binding by scintillat... | Bioorg Med Chem 21: 856-68 (2013) Article DOI: 10.1016/j.bmc.2012.12.016 BindingDB Entry DOI: 10.7270/Q2JD4Z4M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor VII/tissue factor (Homo sapiens (Human)) | BDBM50424075 ((-)-Corydalmine | CHEMBL448891) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 152 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of tissue factor procoagulant activity in LPS-stimulated human THP1 cells preincubated for 1 hr before LPS addition measured after 5 hrs | Bioorg Med Chem 21: 62-9 (2012) Article DOI: 10.1016/j.bmc.2012.11.002 BindingDB Entry DOI: 10.7270/Q2M32X22 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50424075 ((-)-Corydalmine | CHEMBL448891) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 190 | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Agonist activity at 5-HT1A receptor (unknown origin) expressed in HEK293 cells assessed as inhibition of [35S]GTPgammaS binding by scintillation prox... | Bioorg Med Chem 21: 856-68 (2013) Article DOI: 10.1016/j.bmc.2012.12.016 BindingDB Entry DOI: 10.7270/Q2JD4Z4M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||