

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50425045 CHEMBL2312896::CHEMBL2322964::US9402842, 43
SMILES: NCCCn1c2-c3ccccc3C(=O)c2c2ccc(NCC(O)=O)cc2c1=O
InChI Key: InChIKey=ZKDKMZHAOXZKID-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tyrosyl-DNA phosphodiesterase 1 (TDP1) (Homo sapiens (Human)) | BDBM50425045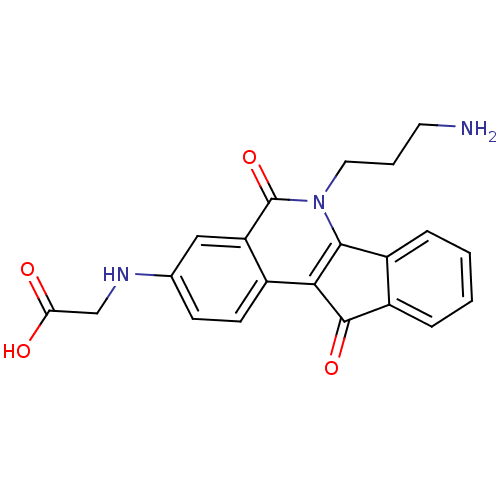 (CHEMBL2312896 | CHEMBL2322964 | US9402842, 43) | PDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Purdue Research Foundation US Patent | Assay Description Tdp1 reactions were performed as recently described. Briefly, a 5'-[32P]-labeled single-stranded DNA oligonucleotide containing a 3'-phosphotyrosine ... | US Patent US8912213 (2014) BindingDB Entry DOI: 10.7270/Q2FN14XB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-alpha (Homo sapiens (Human)) | BDBM50425045 (CHEMBL2312896 | CHEMBL2322964 | US9402842, 43) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 8.40E+3 | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Activation of human RXRalpha activity expressed in COS1 cells after 12 hrs by RXRE-luciferase reporter gene assay | J Med Chem 56: 2581-605 (2013) Article DOI: 10.1021/jm400026k BindingDB Entry DOI: 10.7270/Q2G44RNG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosyl-DNA phosphodiesterase 1 (TDP1) (Homo sapiens (Human)) | BDBM50425045 (CHEMBL2312896 | CHEMBL2322964 | US9402842, 43) | PDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of recombinant Tdp1 (unknown origin) using 5'-[32P]-labeled single-stranded DNA oligonucleotide containing 3'-phosphotyrosine as substrate... | J Med Chem 56: 182-200 (2013) Article DOI: 10.1021/jm3014458 BindingDB Entry DOI: 10.7270/Q2MS3V3Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosyl-DNA phosphodiesterase 1 (TDP1) (Homo sapiens (Human)) | BDBM50425045 (CHEMBL2312896 | CHEMBL2322964 | US9402842, 43) | PDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Purdue Research Foundation US Patent | Assay Description A 5′-[32P]-labeled single-stranded DNA oligonucleotide containing a 3′-phosphotyrosine (N14Y) was generated as described by Dexheimer et ... | US Patent US9402842 (2016) BindingDB Entry DOI: 10.7270/Q2H41Q9R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||