

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50427350 CHEMBL2325740::US10654855, Example 64::US9492453, 64
SMILES: COCCC(NC(=O)C1(N)CCN(CC1)c1ncnc2[nH]ccc12)c1ccc(Cl)cc1
InChI Key: InChIKey=IDQNLUQAYNKBEW-UHFFFAOYSA-N
Data: 7 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RAC-alpha serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM50427350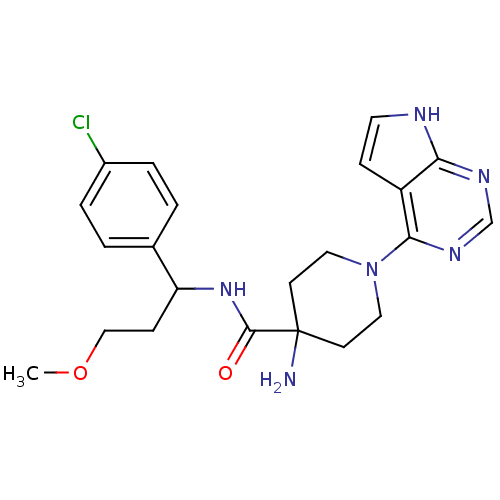 (CHEMBL2325740 | US10654855, Example 64 | US9492453...) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 9.5 | n/a | n/a | n/a | n/a | 7.5 | 25 |
AstraZeneca AB US Patent | Assay Description This assay detects inhibitors of AKT1 (PKBα) kinase activity using Caliper LabChip LC3000. The Caliper off-chip incubation mobility shift assay... | US Patent US9492453 (2016) BindingDB Entry DOI: 10.7270/Q2KH0M83 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-alpha serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM50427350 (CHEMBL2325740 | US10654855, Example 64 | US9492453...) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of recombinant Akt1 (unknown origin) using 5-FAM-labeled peptide as substrate after 1 hr by caliper off-chip incubation mobility shift ass... | J Med Chem 56: 2059-73 (2013) Article DOI: 10.1021/jm301762v BindingDB Entry DOI: 10.7270/Q2QR4ZFN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 2 (Homo sapiens (Human)) | BDBM50427350 (CHEMBL2325740 | US10654855, Example 64 | US9492453...) | PDB MMDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 204 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of recombinant ROCK2 (unknown origin) using 5-FAM-labeled peptide as substrate after 1 hr by caliper off-chip incubation mobility shift as... | J Med Chem 56: 2059-73 (2013) Article DOI: 10.1021/jm301762v BindingDB Entry DOI: 10.7270/Q2QR4ZFN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| AKT1/PPP1CA (Homo sapiens (Human)) | US11236095, Example 64 (CHEMBL2325740 | US10654855, Example 64 | US9492453...) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | n/a | n/a | 9.5 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase AKT2 (Homo sapiens (Human)) | BDBM50427350 (CHEMBL2325740 | US10654855, Example 64 | US9492453...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of recombinant Akt2 (unknown origin) using 5-FAM-labeled peptide as substrate after 1 hr by caliper off-chip incubation mobility shift ass... | J Med Chem 56: 2059-73 (2013) Article DOI: 10.1021/jm301762v BindingDB Entry DOI: 10.7270/Q2QR4ZFN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50427350 (CHEMBL2325740 | US10654855, Example 64 | US9492453...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.37E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human ERG expressed in CHO cells by ionworks assay | J Med Chem 56: 2059-73 (2013) Article DOI: 10.1021/jm301762v BindingDB Entry DOI: 10.7270/Q2QR4ZFN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-alpha serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM50427350 (CHEMBL2325740 | US10654855, Example 64 | US9492453...) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 9.5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca AB US Patent | Assay Description For Echo dosing the solvent was 100% DMSO. A master plate was prepared with 40 ul of 10 mM stock from our Primary Liquid Store in quadrant 1 of a Lab... | US Patent US10654855 (2020) | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase AKT (Homo sapiens (Human)) | BDBM50427350 (CHEMBL2325740 | US10654855, Example 64 | US9492453...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of recombinant Akt3 (unknown origin) using 5-FAM-labeled peptide as substrate after 1 hr by caliper off-chip incubation mobility shift ass... | J Med Chem 56: 2059-73 (2013) Article DOI: 10.1021/jm301762v BindingDB Entry DOI: 10.7270/Q2QR4ZFN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||