

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
null
SMILES: COc1cccc(Nc2nc(N)nc3[nH]c4cccc(Cl)c4c23)c1
InChI Key: InChIKey=WAXJONPSDKJXDR-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Platelet-derived growth factor receptor beta (Homo sapiens (Human)) | BDBM50430567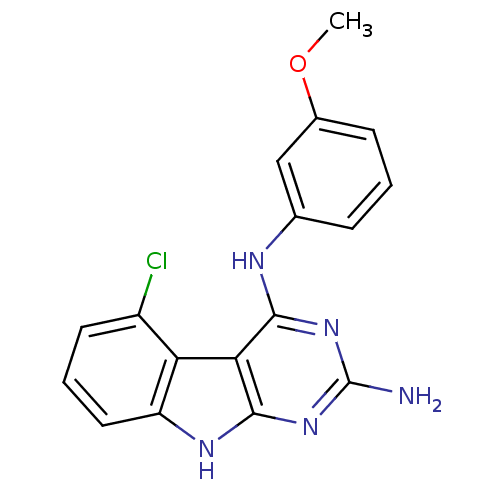 (CHEMBL2337366) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.05E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University Curated by ChEMBL | Assay Description Inhibition of PDGFRbeta in human SF539 cells assessed as inhibition of PDGFR-BB-induced tyrosine phosphorylation incubated for 60 mins prior to EGF-a... | Bioorg Med Chem 21: 1857-64 (2013) Article DOI: 10.1016/j.bmc.2013.01.040 BindingDB Entry DOI: 10.7270/Q2NS0W97 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM50430567 (CHEMBL2337366) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.01E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University Curated by ChEMBL | Assay Description Inhibition of VEGFR2 in human U251 cells assessed as inhibition of VEGF-induced tyrosine phosphorylation incubated for 60 mins prior to VEGF-activati... | Bioorg Med Chem 21: 1857-64 (2013) Article DOI: 10.1016/j.bmc.2013.01.040 BindingDB Entry DOI: 10.7270/Q2NS0W97 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50430567 (CHEMBL2337366) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.29E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University Curated by ChEMBL | Assay Description Inhibition of EGFR in human A431 cells assessed as inhibition of EGF-induced tyrosine phosphorylation incubated for 60 mins prior to EGF-activation m... | Bioorg Med Chem 21: 1857-64 (2013) Article DOI: 10.1016/j.bmc.2013.01.040 BindingDB Entry DOI: 10.7270/Q2NS0W97 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||