

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50431015 CHEMBL2338329::US10889546, FG-4592::US9115085, Compound A Form A::US9340511, 3
SMILES: Cc1nc(C(=O)NCC(O)=O)c(O)c2ccc(Oc3ccccc3)cc12
InChI Key: InChIKey=YOZBGTLTNGAVFU-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prolyl 4-hydroxylase (Paramecium bursaria Chlorella virus 1) | BDBM50431015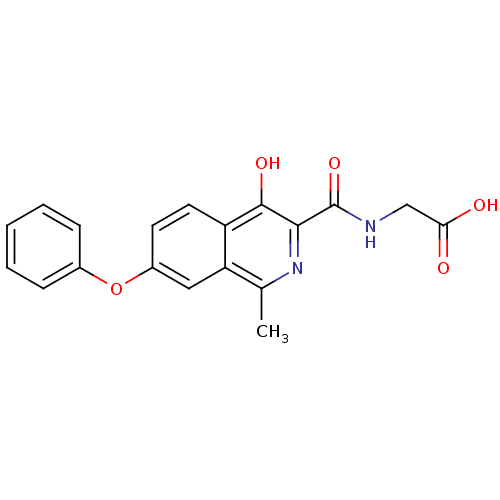 (CHEMBL2338329 | US10889546, FG-4592 | US9115085, C...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | 1.07E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford Curated by ChEMBL | Assay Description Inhibition of N-terminal His6-tagged recombinant Paramecium bursaria chlorella virus 1 CPH expressed in Escherichia coli Rosetta 2 (DE3) cells pre-in... | Bioorg Med Chem 27: 2405-2412 (2019) Article DOI: 10.1016/j.bmc.2019.01.018 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Egl nine homolog 1 (Homo sapiens (Human)) | BDBM50431015 (CHEMBL2338329 | US10889546, FG-4592 | US9115085, C...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Similars | US Patent | n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | 7.4 | n/a |
FibroGen, Inc. US Patent | Assay Description Ketoglutaric acid α-[1-14C]-sodium salt, alpha-ketoglutaric acid sodium salt, and HPLC purified peptide were obtained from commercial sources, ... | US Patent US9340511 (2016) BindingDB Entry DOI: 10.7270/Q28W3C50 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Egl nine homolog 1 (Homo sapiens (Human)) | BDBM50431015 (CHEMBL2338329 | US10889546, FG-4592 | US9115085, C...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Similars | PubMed | n/a | n/a | 591 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Displacement of FITC-HIF-1alpha (556 to 574 residues) from PHD2 (181 to 426 residues) (unknown origin) after 60 mins by fluorescence polarization ass... | ACS Med Chem Lett 6: 1236-40 (2015) BindingDB Entry DOI: 10.7270/Q2RB76G9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-ketoglutarate-dependent dioxygenase FTO (Homo sapiens (Human)) | BDBM50431015 (CHEMBL2338329 | US10889546, FG-4592 | US9115085, C...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 9.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford Curated by ChEMBL | Assay Description Inhibition of human hexahistidine-tagged full-length FTO expressed in Escherichia coli BL21 (DE3) using 3-methylthymidine as substrate assessed as in... | J Med Chem 56: 3680-8 (2013) Article DOI: 10.1021/jm400193d BindingDB Entry DOI: 10.7270/Q21V5GBQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Egl nine homolog 1 (Homo sapiens (Human)) | BDBM50431015 (CHEMBL2338329 | US10889546, FG-4592 | US9115085, C...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 5.74E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of HIF-PHD2 (181 to 426 residues) (unknown origin) using HIF1-alpha (556 to 574 residues) as substrate in presence of 2-OG preincubated fo... | J Med Chem 61: 5332-5349 (2018) Article DOI: 10.1021/acs.jmedchem.8b00549 BindingDB Entry DOI: 10.7270/Q2PN985B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Egl nine homolog 1 (Homo sapiens (Human)) | BDBM50431015 (CHEMBL2338329 | US10889546, FG-4592 | US9115085, C...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 591 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of HIF-PHD2 (181 to 426 residues) (unknown origin) using FITC-HIF1-alpha (556 to 574 residues) as substrate after 60 mins by fluorescence ... | J Med Chem 61: 5332-5349 (2018) Article DOI: 10.1021/acs.jmedchem.8b00549 BindingDB Entry DOI: 10.7270/Q2PN985B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Egl nine homolog 1 (Homo sapiens (Human)) | BDBM50431015 (CHEMBL2338329 | US10889546, FG-4592 | US9115085, C...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Similars | US Patent | n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | 7.4 | n/a |
FIBROGEN, INC. US Patent | Assay Description Ketoglutaric acid alpha -[1-14C]-sodium salt, alpha-ketoglutaric acid sodium salt, and HPLC purified peptide were obtained from commercial sources, e... | US Patent US9115085 (2015) BindingDB Entry DOI: 10.7270/Q29C6W6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Egl nine homolog 1 (Homo sapiens (Human)) | BDBM50431015 (CHEMBL2338329 | US10889546, FG-4592 | US9115085, C...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford Curated by ChEMBL | Assay Description Inhibition of recombinant human PHD2 using 2OG as substrate and Fe2 as co-factor assessed as hydroxylation incubated for 15 mins in presence of L-asc... | Bioorg Med Chem 27: 2405-2412 (2019) Article DOI: 10.1016/j.bmc.2019.01.018 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl 4-hydroxylase (Paramecium bursaria Chlorella virus 1) | BDBM50431015 (CHEMBL2338329 | US10889546, FG-4592 | US9115085, C...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford Curated by ChEMBL | Assay Description Inhibition of N-terminal His6-tagged recombinant Paramecium bursaria chlorella virus 1 CPH expressed in Escherichia coli Rosetta 2 (DE3) cells pre-in... | Bioorg Med Chem 27: 2405-2412 (2019) Article DOI: 10.1016/j.bmc.2019.01.018 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hypoxia-inducible factor 1 (FIH) (Homo sapiens (Human)) | BDBM50431015 (CHEMBL2338329 | US10889546, FG-4592 | US9115085, C...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford Curated by ChEMBL | Assay Description Inhibition of recombinant human FIH using 2OG as substrate and Fe2 as co-factor assessed as hydroxylation incubated for 15 mins in presence of L-asco... | Bioorg Med Chem 27: 2405-2412 (2019) Article DOI: 10.1016/j.bmc.2019.01.018 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl 3-hydroxylase OGFOD1 (Homo sapiens) | BDBM50431015 (CHEMBL2338329 | US10889546, FG-4592 | US9115085, C...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | <1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford Curated by ChEMBL | Assay Description Inhibition of recombinant human OGFOD1 using 2OG as substrate and Fe2 as co-factor assessed as hydroxylation incubated for 15 mins in presence of L-a... | Bioorg Med Chem 27: 2405-2412 (2019) Article DOI: 10.1016/j.bmc.2019.01.018 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Egl nine homolog 1 (Homo sapiens (Human)) | BDBM50431015 (CHEMBL2338329 | US10889546, FG-4592 | US9115085, C...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 575 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of PHD2 (181 to 426 residues) (unknown origin) using FITC-HIF1alpha (556 to 574 residues) as substrate after 60 mins by fluorescence polar... | J Med Chem 62: 7583-7588 (2019) Article DOI: 10.1021/acs.jmedchem.9b00688 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Egl nine homolog 1 (Homo sapiens (Human)) | BDBM50431015 (CHEMBL2338329 | US10889546, FG-4592 | US9115085, C...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Similars | US Patent | n/a | n/a | 599 | n/a | n/a | n/a | n/a | n/a | n/a |
Jiangsu Hengrui Medicine Co., Ltd. US Patent | Assay Description The EGLN-1 (or EGLN-3) enzyme activity is determined using mass spectrometry (matrix-assisted laser desorption ionization, time-of-flight MS, MALD... | US Patent US10889546 (2021) | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||