 Found 6 hits for monomerid = 50432306
Found 6 hits for monomerid = 50432306 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50432306
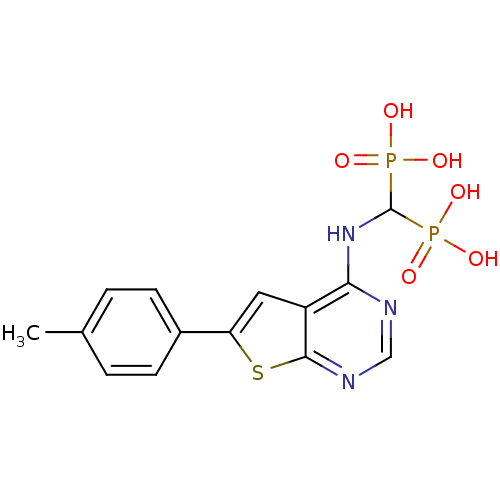
(CHEMBL2347862)Show SMILES Cc1ccc(cc1)-c1cc2c(NC(P(O)(O)=O)P(O)(O)=O)ncnc2s1 Show InChI InChI=1S/C14H15N3O6P2S/c1-8-2-4-9(5-3-8)11-6-10-12(15-7-16-13(10)26-11)17-14(24(18,19)20)25(21,22)23/h2-7,14H,1H3,(H,15,16,17)(H2,18,19,20)(H2,21,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University
Curated by ChEMBL
| Assay Description
Allosteric inhibition of human recombinant FPPS using GPP and [3H]IPP as substrate incubated with enzyme for 10 mins prior to substrate addition by l... |
J Med Chem 57: 5764-76 (2014)
Article DOI: 10.1021/jm500629e
BindingDB Entry DOI: 10.7270/Q2GH9KJR |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50432306

(CHEMBL2347862)Show SMILES Cc1ccc(cc1)-c1cc2c(NC(P(O)(O)=O)P(O)(O)=O)ncnc2s1 Show InChI InChI=1S/C14H15N3O6P2S/c1-8-2-4-9(5-3-8)11-6-10-12(15-7-16-13(10)26-11)17-14(24(18,19)20)25(21,22)23/h2-7,14H,1H3,(H,15,16,17)(H2,18,19,20)(H2,21,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University
Curated by ChEMBL
| Assay Description
Inhibition of FPPS (unknown origin) |
J Med Chem 57: 5764-76 (2014)
Article DOI: 10.1021/jm500629e
BindingDB Entry DOI: 10.7270/Q2GH9KJR |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50432306

(CHEMBL2347862)Show SMILES Cc1ccc(cc1)-c1cc2c(NC(P(O)(O)=O)P(O)(O)=O)ncnc2s1 Show InChI InChI=1S/C14H15N3O6P2S/c1-8-2-4-9(5-3-8)11-6-10-12(15-7-16-13(10)26-11)17-14(24(18,19)20)25(21,22)23/h2-7,14H,1H3,(H,15,16,17)(H2,18,19,20)(H2,21,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 390 | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant N-terminal His6-tagged FPPS transfected in Escherichia coli BL21 (DE3) cells using GPP and [3H]-IPP as substrate incu... |
Bioorg Med Chem 21: 2229-40 (2013)
Article DOI: 10.1016/j.bmc.2013.02.006
BindingDB Entry DOI: 10.7270/Q2GM88PG |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50432306

(CHEMBL2347862)Show SMILES Cc1ccc(cc1)-c1cc2c(NC(P(O)(O)=O)P(O)(O)=O)ncnc2s1 Show InChI InChI=1S/C14H15N3O6P2S/c1-8-2-4-9(5-3-8)11-6-10-12(15-7-16-13(10)26-11)17-14(24(18,19)20)25(21,22)23/h2-7,14H,1H3,(H,15,16,17)(H2,18,19,20)(H2,21,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant FPPS using GPP/[3H]IPP as substrate incubated for 10 mins prior to substrate addition measured after 8 mins by scinti... |
J Med Chem 56: 7939-50 (2013)
Article DOI: 10.1021/jm400946f
BindingDB Entry DOI: 10.7270/Q20V8F6B |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50432306

(CHEMBL2347862)Show SMILES Cc1ccc(cc1)-c1cc2c(NC(P(O)(O)=O)P(O)(O)=O)ncnc2s1 Show InChI InChI=1S/C14H15N3O6P2S/c1-8-2-4-9(5-3-8)11-6-10-12(15-7-16-13(10)26-11)17-14(24(18,19)20)25(21,22)23/h2-7,14H,1H3,(H,15,16,17)(H2,18,19,20)(H2,21,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 390 | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant FPPS using GPP/[3H]IPP as substrate incubated for 10 mins prior to substrate addition by scintillation counting analy... |
J Med Chem 56: 7939-50 (2013)
Article DOI: 10.1021/jm400946f
BindingDB Entry DOI: 10.7270/Q20V8F6B |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50432306

(CHEMBL2347862)Show SMILES Cc1ccc(cc1)-c1cc2c(NC(P(O)(O)=O)P(O)(O)=O)ncnc2s1 Show InChI InChI=1S/C14H15N3O6P2S/c1-8-2-4-9(5-3-8)11-6-10-12(15-7-16-13(10)26-11)17-14(24(18,19)20)25(21,22)23/h2-7,14H,1H3,(H,15,16,17)(H2,18,19,20)(H2,21,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | 3.20E+3 | n/a | n/a | n/a | n/a | n/a |
McGill University
Curated by ChEMBL
| Assay Description
Binding affinity to human FPPS by isothermal titration colorimetry in absence of Mg2+ ions |
J Med Chem 57: 5764-76 (2014)
Article DOI: 10.1021/jm500629e
BindingDB Entry DOI: 10.7270/Q2GH9KJR |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data