

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50433839 CHEMBL2382405
SMILES: CCC(Sc1nc(-c2cccc(OC)c2)c(C#N)c(=O)[nH]1)C(=O)Nc1ccc(cc1)S(N)(=O)=O
InChI Key: InChIKey=DLDDCGAZVYQHHN-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50433839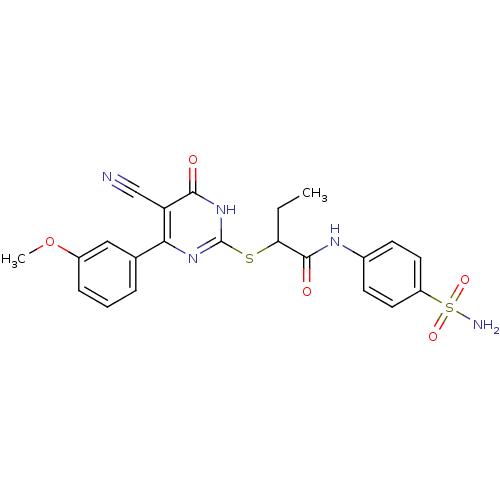 (CHEMBL2382405) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 6.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibition of human carbonic anhydrase 2 preincubated for 6 hrs at 4 degC measured for 10 to 100 secs by stopped-flow carbon dioxide hydration assay | Bioorg Med Chem 24: 3643-8 (2016) BindingDB Entry DOI: 10.7270/Q27S7QQG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 12 (Homo sapiens (Human)) | BDBM50433839 (CHEMBL2382405) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibition of human carbonic anhydrase 12 preincubated for 6 hrs at 4 degC measured for 10 to 100 secs by stopped-flow carbon dioxide hydration assay | Bioorg Med Chem 24: 3643-8 (2016) BindingDB Entry DOI: 10.7270/Q27S7QQG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 9 (Homo sapiens (Human)) | BDBM50433839 (CHEMBL2382405) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibition of human carbonic anhydrase 9 catalytic domain preincubated for 6 hrs at 4 degC measured for 10 to 100 secs by stopped-flow carbon dioxide... | Bioorg Med Chem 24: 3643-8 (2016) BindingDB Entry DOI: 10.7270/Q27S7QQG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM50433839 (CHEMBL2382405) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibition of human carbonic anhydrase 1 preincubated for 6 hrs at 4 degC measured for 10 to 100 secs by stopped-flow carbon dioxide hydration assay | Bioorg Med Chem 24: 3643-8 (2016) BindingDB Entry DOI: 10.7270/Q27S7QQG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lactate dehydrogenase A (LDHA) (Homo sapiens (Human)) | BDBM50433839 (CHEMBL2382405) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 8.90E+3 | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Binding affinity to human recombinant carboxy-terminal avi-tagged LDHA (1 to 331) expressed in Escherichia coli by surface plasmon resonance analysis... | Bioorg Med Chem Lett 23: 3186-94 (2013) Article DOI: 10.1016/j.bmcl.2013.04.001 BindingDB Entry DOI: 10.7270/Q29C6ZTT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lactate dehydrogenase A (LDHA) (Homo sapiens (Human)) | BDBM50433839 (CHEMBL2382405) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Inhibition of human recombinant carboxy-terminal his-tagged LDHA (1 to 331) expressed in Escherichia coli using pyruvate as substrate after 10 mins b... | Bioorg Med Chem Lett 23: 3186-94 (2013) Article DOI: 10.1016/j.bmcl.2013.04.001 BindingDB Entry DOI: 10.7270/Q29C6ZTT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lactate dehydrogenase B (LDHB) (Homo sapiens (Human)) | BDBM50433839 (CHEMBL2382405) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Inhibition of human recombinant carboxy-terminal his-tagged LDHB (1 to 333) expressed in insect cells using pyruvate as substrate after 10 mins by UV... | Bioorg Med Chem Lett 23: 3186-94 (2013) Article DOI: 10.1016/j.bmcl.2013.04.001 BindingDB Entry DOI: 10.7270/Q29C6ZTT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||