

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50437951 CHEMBL2408922::US9403843, 24a
SMILES: CC(C)C[C@H](NC(=O)[C@H]1O[C@@H]1C(O)=O)C(=O)Nc1nc(cs1)-c1ccc(F)cc1
InChI Key: InChIKey=WVMZJHSGEWZNEO-QEJZJMRPSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Papain (Carica papaya) | BDBM50437951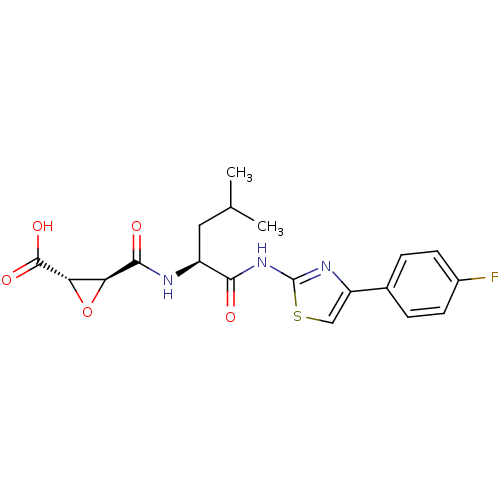 (CHEMBL2408922 | US9403843, 24a) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 1.62E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois College of Pharmacy Curated by ChEMBL | Assay Description Inhibition of papain after using SucLLVYAMC as substrate by FRET assay | J Med Chem 56: 6054-68 (2013) Article DOI: 10.1021/jm4006719 BindingDB Entry DOI: 10.7270/Q2VT1TG7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calpain-1 (Sus scrofa (pig)) | BDBM50437951 (CHEMBL2408922 | US9403843, 24a) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | US Patent | 6.00E+3 | -7.24 | n/a | n/a | n/a | n/a | n/a | 7.6 | 30 |
THE TRUSTEES OF COLUMBIA UNIVERSITY IN THE CITY OF NEW YORK; THE BOARD OF TRUSTEES OF THE UNIVERSITY OF ILLINOIS US Patent | Assay Description Full length porcine calpain (156 nM), or papain (236 pM) was added to a solution of 100 mM NaCl, 50 mM HEPES, pH 7.6, 1 mM TCEP, 30 μM Suc-LLVY-AM... | US Patent US9403843 (2016) BindingDB Entry DOI: 10.7270/Q2KP8124 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calpain-1 (Sus scrofa (pig)) | BDBM50437951 (CHEMBL2408922 | US9403843, 24a) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 6.02E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois College of Pharmacy Curated by ChEMBL | Assay Description Inhibition of pig full length Cal1 after using SucLLVYAMC as substrate by FRET assay | J Med Chem 56: 6054-68 (2013) Article DOI: 10.1021/jm4006719 BindingDB Entry DOI: 10.7270/Q2VT1TG7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calpain1 (Homo sapiens (Human)) | BDBM50437951 (CHEMBL2408922 | US9403843, 24a) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 250 | n/a | n/a | n/a | n/a | 7.4 | n/a |
THE TRUSTEES OF COLUMBIA UNIVERSITY IN THE CITY OF NEW YORK; THE BOARD OF TRUSTEES OF THE UNIVERSITY OF ILLINOIS US Patent | Assay Description The Calbiochem InnoZyme activity kit was used for measuring the inhibition effect inhibitors on human erythrocyte calpain 1 activity. A calpain FRET ... | US Patent US9403843 (2016) BindingDB Entry DOI: 10.7270/Q2KP8124 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calpain1 (Homo sapiens (Human)) | BDBM50437951 (CHEMBL2408922 | US9403843, 24a) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 50 | n/a | n/a | n/a | n/a | 7.4 | n/a |
THE TRUSTEES OF COLUMBIA UNIVERSITY IN THE CITY OF NEW YORK; THE BOARD OF TRUSTEES OF THE UNIVERSITY OF ILLINOIS US Patent | Assay Description The Calbiochem InnoZyme activity kit was used for measuring the inhibition effect inhibitors on human erythrocyte calpain 1 activity. A calpain FRET ... | US Patent US9403843 (2016) BindingDB Entry DOI: 10.7270/Q2KP8124 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calpain1 (Homo sapiens (Human)) | BDBM50437951 (CHEMBL2408922 | US9403843, 24a) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 30 | n/a | n/a | n/a | n/a | 7.4 | n/a |
THE TRUSTEES OF COLUMBIA UNIVERSITY IN THE CITY OF NEW YORK; THE BOARD OF TRUSTEES OF THE UNIVERSITY OF ILLINOIS US Patent | Assay Description The Calbiochem InnoZyme activity kit was used for measuring the inhibition effect inhibitors on human erythrocyte calpain 1 activity. A calpain FRET ... | US Patent US9403843 (2016) BindingDB Entry DOI: 10.7270/Q2KP8124 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||