

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50438910 CHEMBL1450796::US9073941, 618
SMILES: Cc1ccc(cc1)-c1nn(C)c2nc(=O)n(C)c(=O)c2n1
InChI Key: InChIKey=ADLBKNFHIOUYGV-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| tyrosyl-DNA phosphodiesterase 2 (Homo sapiens (Human)) | BDBM50438910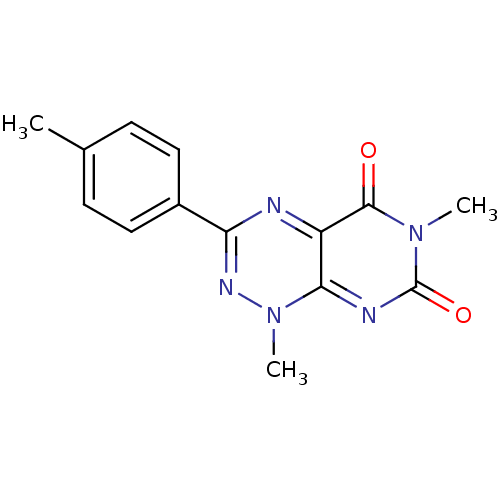 (CHEMBL1450796 | US9073941, 618) | PDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 290 | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibition of TDP2 (unknown origin) using 4-nitrophenyl phenylphosphonate as substrate after 60 mins | J Med Chem 56: 6352-70 (2013) Article DOI: 10.1021/jm400568p BindingDB Entry DOI: 10.7270/Q2N017ZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apurinic-apyrimidinic endonuclease 1 (APE-1) (Homo sapiens (Human)) | BDBM50438910 (CHEMBL1450796 | US9073941, 618) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibition of APE-1 (unknown origin) using double-stranded AP-site containing DNA as substrate after 30 mins by fluorescence assay | J Med Chem 56: 6352-70 (2013) Article DOI: 10.1021/jm400568p BindingDB Entry DOI: 10.7270/Q2N017ZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| NAD-dependent deacetylase sirtuin 2 (Homo sapiens (Human)) | BDBM50438910 (CHEMBL1450796 | US9073941, 618) | PDB MMDB NCI pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Guru Jambheshwar University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of human SIRT2 using biotin labeled histone H4 peptide with an acetylated lysine 4 as substrate measured after 1 hr by luminescence analys... | Eur J Med Chem 119: 45-69 (2016) Article DOI: 10.1016/j.ejmech.2016.04.063 BindingDB Entry DOI: 10.7270/Q2VH5QTW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pyruvate dehydrogenase (PDH) (Mycobacterium tuberculosis) | BDBM50438910 (CHEMBL1450796 | US9073941, 618) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <40 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Academia Sinica US Patent | Assay Description Mtb PDH (Lpd+DlaT+AceE) is provided by Dr. Bryk Ruslana. The assay was performed in a manner similar to that described in Bryk et al., Biochemistry (... | US Patent US9073941 (2015) BindingDB Entry DOI: 10.7270/Q2HX1BGC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| NAD-dependent deacetylase sirtuin 1 (Homo sapiens (Human)) | BDBM50438910 (CHEMBL1450796 | US9073941, 618) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Guru Jambheshwar University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of human SIRT1 using biotinylated peptide with an acetylated FLAG sequence as substrate measured after 3 hr in presence of NAD+ by TR-FRET... | Eur J Med Chem 119: 45-69 (2016) Article DOI: 10.1016/j.ejmech.2016.04.063 BindingDB Entry DOI: 10.7270/Q2VH5QTW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lipoamide Dehydrogenase (Lpd) (Mycobacterium tuberculosis) | BDBM50438910 (CHEMBL1450796 | US9073941, 618) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <40 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Academia Sinica US Patent | Assay Description The assay was performed in a manner similar to that described in Bryk et al., Biochemistry (2010) 49:1616-1627 and modified for an online robotics sc... | US Patent US9073941 (2015) BindingDB Entry DOI: 10.7270/Q2HX1BGC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||