

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50441571 CHEMBL2436982::US10485800, Example 18::US9814704, Example 18
SMILES: COc1cc(cc(OC)c1OC)-c1cnc2[nH]cc(-c3ccc(N)nc3)c2c1
InChI Key: InChIKey=DFBYFJSXUCUKLA-UHFFFAOYSA-N
Data: 7 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mitogen-activated protein kinase kinase kinase 11 (Homo sapiens (Human)) | BDBM50441571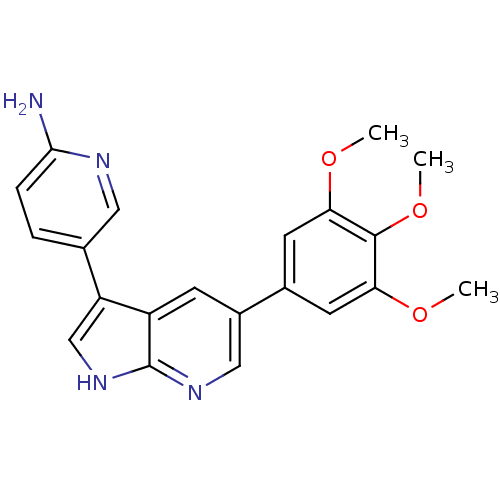 (CHEMBL2436982 | US10485800, Example 18 | US9814704...) | PDB NCI pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Califia Bio Inc. Curated by ChEMBL | Assay Description Inhibition of MLK3 (unknown origin) after 20 mins by scintillation counting analysis in presence of [33P]-ATP | J Med Chem 56: 8032-48 (2013) Article DOI: 10.1021/jm401094t BindingDB Entry DOI: 10.7270/Q2P270KS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine-rich repeat serine/threonine-protein kinase 2 (LRRK2) (Homo sapiens (Human)) | BDBM50441571 (CHEMBL2436982 | US10485800, Example 18 | US9814704...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Califia Bio Inc. Curated by ChEMBL | Assay Description Inhibition of human wild type LRRK2 by qPCR analysis | J Med Chem 56: 8032-48 (2013) Article DOI: 10.1021/jm401094t BindingDB Entry DOI: 10.7270/Q2P270KS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase kinase kinase 11 (Mus musculus) | BDBM50441571 (CHEMBL2436982 | US10485800, Example 18 | US9814704...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Califia Bio Inc. Curated by ChEMBL | Assay Description Inhibition of MLK3 in mouse BV2 cells assessed as inhibition of LPS-induced TNFalpha release after 8 hrs by ELISA | J Med Chem 56: 8032-48 (2013) Article DOI: 10.1021/jm401094t BindingDB Entry DOI: 10.7270/Q2P270KS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase kinase kinase 11 (Homo sapiens (Human)) | BDBM50441571 (CHEMBL2436982 | US10485800, Example 18 | US9814704...) | PDB NCI pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Rochester; Board of Regents of the University of Nebraska US Patent | Assay Description 200 ng (130 nM) MLK3 (Dundee, DU8313) was incubated with 1 μM inactive MKK7b (Dundee, DU703) in the presence of 2 μM cold ATP (Km) and 0.5 ... | US Patent US10485800 (2019) BindingDB Entry DOI: 10.7270/Q22B91DW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50441571 (CHEMBL2436982 | US10485800, Example 18 | US9814704...) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Califia Bio Inc. Curated by ChEMBL | Assay Description Inhibition of CYP2D6 in human liver microsomes assessed as inhibition of diclofenac metabolism by HPLC/MS analysis | J Med Chem 56: 8032-48 (2013) Article DOI: 10.1021/jm401094t BindingDB Entry DOI: 10.7270/Q2P270KS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase kinase kinase 11 (Homo sapiens (Human)) | BDBM50441571 (CHEMBL2436982 | US10485800, Example 18 | US9814704...) | PDB NCI pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Rochester US Patent | Assay Description 200 ng (130 nM) MLK3 (Dundee, DU8313) was incubated with 1 μM inactive MKK7b (Dundee, DU703) in the presence of 2 μM cold ATP (Km) and 0.5 ... | US Patent US9814704 (2017) BindingDB Entry DOI: 10.7270/Q27H1MRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50441571 (CHEMBL2436982 | US10485800, Example 18 | US9814704...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Califia Bio Inc. Curated by ChEMBL | Assay Description Inhibition of CYP2C9 in human liver microsomes assessed as inhibition of midazolam metabolism by HPLC/MS analysis | J Med Chem 56: 8032-48 (2013) Article DOI: 10.1021/jm401094t BindingDB Entry DOI: 10.7270/Q2P270KS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||