

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50442610 CHEMBL2441428
SMILES: Clc1cc(CCN2CCN(CC2)C(=O)Cc2ccc(cc2)-n2cnnn2)cc2COC(=O)c12
InChI Key: InChIKey=LXARJHMGHFCGNE-UHFFFAOYSA-N
Data: 1 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ATP-sensitive inward rectifier potassium channel 1 (Homo sapiens (Human)) | BDBM50442610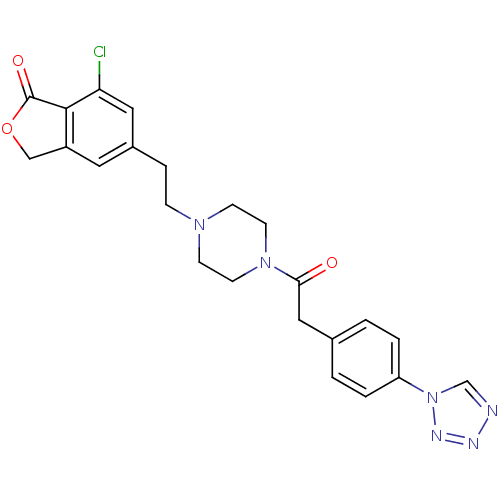 (CHEMBL2441428) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 830 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human ROMK channel-mediated 86Rb+ flux expressed in CHO cells after 35 mins by scintillation counting analysis | Bioorg Med Chem Lett 23: 5829-32 (2013) Article DOI: 10.1016/j.bmcl.2013.08.104 BindingDB Entry DOI: 10.7270/Q28K7BHG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||