

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50443545 CHEMBL3091683
SMILES: C(CCN(C[C@H]1Cc2ccccc2CN1)[C@H]1CCCc2cccnc12)CNCc1ccccn1
InChI Key: InChIKey=DBUIDMKLHBXFJG-IZLXSDGUSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C-X-C chemokine receptor type 4 (CXCR4) (Homo sapiens (Human)) | BDBM50443545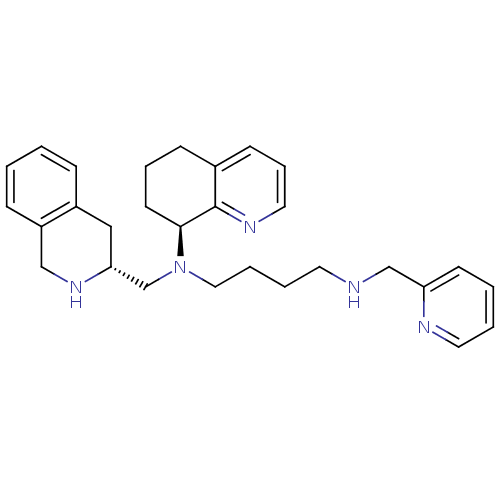 (CHEMBL3091683) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description Antagonist activity at CXCR4 in human Chem-1 cells assessed as inhibition of SDF-1alpha-mediated calcium flux preincubated for 10 mins by FLIPR assay | ACS Med Chem Lett 4: 1025-30 (2013) Article DOI: 10.1021/ml400183q BindingDB Entry DOI: 10.7270/Q2ZS2Z0K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (CXCR4) (Homo sapiens (Human)) | BDBM50443545 (CHEMBL3091683) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description Antagonist activity at CXCR4 in human MAGI-CCR5 cells assessed as inhibition of HIV-1 3B entry after 2 to 6 days by beta-galactosidase reporter gene ... | ACS Med Chem Lett 4: 1025-30 (2013) Article DOI: 10.1021/ml400183q BindingDB Entry DOI: 10.7270/Q2ZS2Z0K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50443545 (CHEMBL3091683) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description Antagonist activity at human CXCR4 in CCRF-CEM cells assessed as decrease in SDF-1alpha stimulated Ca2+ flux preincubated for 25 mins followed by SDF... | J Med Chem 61: 946-979 (2018) Article DOI: 10.1021/acs.jmedchem.7b01420 BindingDB Entry DOI: 10.7270/Q2KH0QTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50443545 (CHEMBL3091683) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description Inhibition of CYP3A4 (unknown origin) | J Med Chem 61: 946-979 (2018) Article DOI: 10.1021/acs.jmedchem.7b01420 BindingDB Entry DOI: 10.7270/Q2KH0QTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50443545 (CHEMBL3091683) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 350 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description Inhibition of recombinant human CYP2D6 expressed in microsomes of insect cells using AMMC as substrate preincubated for 30 mins followed by NADP addi... | J Med Chem 61: 946-979 (2018) Article DOI: 10.1021/acs.jmedchem.7b01420 BindingDB Entry DOI: 10.7270/Q2KH0QTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50443545 (CHEMBL3091683) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description Antagonist activity at human CXCR4 in CCRF-CEM cells assessed as decrease in SDF-1alpha stimulated Ca2+ flux preincubated for 25 mins followed by SDF... | J Med Chem 61: 946-979 (2018) Article DOI: 10.1021/acs.jmedchem.7b01420 BindingDB Entry DOI: 10.7270/Q2KH0QTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||