 Found 11 hits for monomerid = 50443628
Found 11 hits for monomerid = 50443628 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Serine/threonine-protein kinase Sgk1
(Homo sapiens (Human)) | BDBM50443628
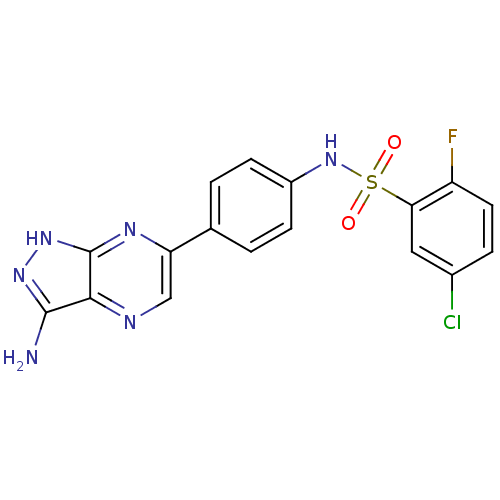
(CHEMBL3092468 | US9174993, 35 | US9221828, 35)Show SMILES Nc1n[nH]c2nc(cnc12)-c1ccc(NS(=O)(=O)c2cc(Cl)ccc2F)cc1 Show InChI InChI=1S/C17H12ClFN6O2S/c18-10-3-6-12(19)14(7-10)28(26,27)25-11-4-1-9(2-5-11)13-8-21-15-16(20)23-24-17(15)22-13/h1-8,25H,(H3,20,22,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
SANOFI
US Patent
| Assay Description
The compounds were tested for serum and glucocorticoid-regulated kinase 1 (SGK-1) inhibitory activity in a substrate phosphorylation assay designed t... |
US Patent US9174993 (2015)
BindingDB Entry DOI: 10.7270/Q2MK6BPF |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase Sgk1
(Homo sapiens (Human)) | BDBM50443628

(CHEMBL3092468 | US9174993, 35 | US9221828, 35)Show SMILES Nc1n[nH]c2nc(cnc12)-c1ccc(NS(=O)(=O)c2cc(Cl)ccc2F)cc1 Show InChI InChI=1S/C17H12ClFN6O2S/c18-10-3-6-12(19)14(7-10)28(26,27)25-11-4-1-9(2-5-11)13-8-21-15-16(20)23-24-17(15)22-13/h1-8,25H,(H3,20,22,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.4 | 24 |
SANOFI
US Patent
| Assay Description
The enzyme reaction was carried out in a buffer containing 25 mM
Tris-HCl (pH 7.4), 5 mM MgCl2, 2 mM MnCl2, 2 mM DTT, and 0.03% bovine
serum albumi... |
US Patent US9221828 (2015)
BindingDB Entry DOI: 10.7270/Q2GX49DW |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase Sgk1
(Homo sapiens (Human)) | BDBM50443628

(CHEMBL3092468 | US9174993, 35 | US9221828, 35)Show SMILES Nc1n[nH]c2nc(cnc12)-c1ccc(NS(=O)(=O)c2cc(Cl)ccc2F)cc1 Show InChI InChI=1S/C17H12ClFN6O2S/c18-10-3-6-12(19)14(7-10)28(26,27)25-11-4-1-9(2-5-11)13-8-21-15-16(20)23-24-17(15)22-13/h1-8,25H,(H3,20,22,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi R&D
Curated by ChEMBL
| Assay Description
Inhibition of human SGK1 using fluo-5(6)-carboxyfluorescein)-RPRAATF-NH2 fluorescently labeled peptide by substrate phosphorylation assay in presence... |
ACS Med Chem Lett 6: 73-8 (2015)
Article DOI: 10.1021/ml5003376
BindingDB Entry DOI: 10.7270/Q28S4RJT |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase Sgk2
(Homo sapiens (Human)) | BDBM50443628

(CHEMBL3092468 | US9174993, 35 | US9221828, 35)Show SMILES Nc1n[nH]c2nc(cnc12)-c1ccc(NS(=O)(=O)c2cc(Cl)ccc2F)cc1 Show InChI InChI=1S/C17H12ClFN6O2S/c18-10-3-6-12(19)14(7-10)28(26,27)25-11-4-1-9(2-5-11)13-8-21-15-16(20)23-24-17(15)22-13/h1-8,25H,(H3,20,22,23,24) | Reactome pathway
KEGG
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 128 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi R&D
Curated by ChEMBL
| Assay Description
Inhibition of human SGK2 in presence of 500 uM ATP |
ACS Med Chem Lett 6: 73-8 (2015)
Article DOI: 10.1021/ml5003376
BindingDB Entry DOI: 10.7270/Q28S4RJT |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase Sgk3
(Homo sapiens (Human)) | BDBM50443628

(CHEMBL3092468 | US9174993, 35 | US9221828, 35)Show SMILES Nc1n[nH]c2nc(cnc12)-c1ccc(NS(=O)(=O)c2cc(Cl)ccc2F)cc1 Show InChI InChI=1S/C17H12ClFN6O2S/c18-10-3-6-12(19)14(7-10)28(26,27)25-11-4-1-9(2-5-11)13-8-21-15-16(20)23-24-17(15)22-13/h1-8,25H,(H3,20,22,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi R&D
Curated by ChEMBL
| Assay Description
Inhibition of human SGK3 in presence of 500 uM ATP |
ACS Med Chem Lett 6: 73-8 (2015)
Article DOI: 10.1021/ml5003376
BindingDB Entry DOI: 10.7270/Q28S4RJT |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase Sgk1
(Homo sapiens (Human)) | BDBM50443628

(CHEMBL3092468 | US9174993, 35 | US9221828, 35)Show SMILES Nc1n[nH]c2nc(cnc12)-c1ccc(NS(=O)(=O)c2cc(Cl)ccc2F)cc1 Show InChI InChI=1S/C17H12ClFN6O2S/c18-10-3-6-12(19)14(7-10)28(26,27)25-11-4-1-9(2-5-11)13-8-21-15-16(20)23-24-17(15)22-13/h1-8,25H,(H3,20,22,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Temple University School of Pharmacy
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human SGK-1 expressed in baculovirus expression system using (5(6)-carboxyfluorescein)-RPRAATF-NH2 as substrate preincubate... |
ACS Med Chem Lett 4: 1022-4 (2013)
Article DOI: 10.1021/ml400389a
BindingDB Entry DOI: 10.7270/Q2XS5WVS |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50443628

(CHEMBL3092468 | US9174993, 35 | US9221828, 35)Show SMILES Nc1n[nH]c2nc(cnc12)-c1ccc(NS(=O)(=O)c2cc(Cl)ccc2F)cc1 Show InChI InChI=1S/C17H12ClFN6O2S/c18-10-3-6-12(19)14(7-10)28(26,27)25-11-4-1-9(2-5-11)13-8-21-15-16(20)23-24-17(15)22-13/h1-8,25H,(H3,20,22,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi R&D
Curated by ChEMBL
| Assay Description
Inhibition of human CYP3A4 midazolam site in human liver microsomes using midazolam as substrate by LC-MS/MS analysis |
ACS Med Chem Lett 6: 73-8 (2015)
Article DOI: 10.1021/ml5003376
BindingDB Entry DOI: 10.7270/Q28S4RJT |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50443628

(CHEMBL3092468 | US9174993, 35 | US9221828, 35)Show SMILES Nc1n[nH]c2nc(cnc12)-c1ccc(NS(=O)(=O)c2cc(Cl)ccc2F)cc1 Show InChI InChI=1S/C17H12ClFN6O2S/c18-10-3-6-12(19)14(7-10)28(26,27)25-11-4-1-9(2-5-11)13-8-21-15-16(20)23-24-17(15)22-13/h1-8,25H,(H3,20,22,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.86E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi R&D
Curated by ChEMBL
| Assay Description
Inhibition of human CYP3A4 testosterone site in human liver microsomes using midazolam as substrate by LC-MS/MS analysis |
ACS Med Chem Lett 6: 73-8 (2015)
Article DOI: 10.1021/ml5003376
BindingDB Entry DOI: 10.7270/Q28S4RJT |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase Sgk1
(Homo sapiens (Human)) | BDBM50443628

(CHEMBL3092468 | US9174993, 35 | US9221828, 35)Show SMILES Nc1n[nH]c2nc(cnc12)-c1ccc(NS(=O)(=O)c2cc(Cl)ccc2F)cc1 Show InChI InChI=1S/C17H12ClFN6O2S/c18-10-3-6-12(19)14(7-10)28(26,27)25-11-4-1-9(2-5-11)13-8-21-15-16(20)23-24-17(15)22-13/h1-8,25H,(H3,20,22,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi R&D
Curated by ChEMBL
| Assay Description
Inhibition of human SGK1 using fluo-5(6)-carboxyfluorescein)-RPRAATF-NH2 fluorescently labeled peptide by substrate phosphorylation assay in presence... |
ACS Med Chem Lett 6: 73-8 (2015)
Article DOI: 10.1021/ml5003376
BindingDB Entry DOI: 10.7270/Q28S4RJT |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase Sgk1
(Homo sapiens (Human)) | BDBM50443628

(CHEMBL3092468 | US9174993, 35 | US9221828, 35)Show SMILES Nc1n[nH]c2nc(cnc12)-c1ccc(NS(=O)(=O)c2cc(Cl)ccc2F)cc1 Show InChI InChI=1S/C17H12ClFN6O2S/c18-10-3-6-12(19)14(7-10)28(26,27)25-11-4-1-9(2-5-11)13-8-21-15-16(20)23-24-17(15)22-13/h1-8,25H,(H3,20,22,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 690 | n/a | n/a | n/a | n/a | n/a | n/a |
Temple University School of Pharmacy
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human SGK1 (S61 to L431, S422D) expressed in human U20S cells assessed as inhibition of GSK3beta phosphorylation after 6 hr... |
ACS Med Chem Lett 4: 1022-4 (2013)
Article DOI: 10.1021/ml400389a
BindingDB Entry DOI: 10.7270/Q2XS5WVS |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase Sgk1
(Homo sapiens (Human)) | BDBM50443628

(CHEMBL3092468 | US9174993, 35 | US9221828, 35)Show SMILES Nc1n[nH]c2nc(cnc12)-c1ccc(NS(=O)(=O)c2cc(Cl)ccc2F)cc1 Show InChI InChI=1S/C17H12ClFN6O2S/c18-10-3-6-12(19)14(7-10)28(26,27)25-11-4-1-9(2-5-11)13-8-21-15-16(20)23-24-17(15)22-13/h1-8,25H,(H3,20,22,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 690 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi R&D
Curated by ChEMBL
| Assay Description
Inhibition of SGK1 in human U2OS cells assessed as phosphorylation of GSK3beta |
ACS Med Chem Lett 6: 73-8 (2015)
Article DOI: 10.1021/ml5003376
BindingDB Entry DOI: 10.7270/Q28S4RJT |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data