

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50445044 CHEMBL3098765::US10195186, Example 36::US9682967, 34
SMILES: Cc1nccc(NC(=O)c2ccc(Br)cc2C(O)=O)n1
InChI Key: InChIKey=XPTZYUYHLHWETQ-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Neurotensin receptor 3 (Homo sapiens (Human)) | BDBM50445044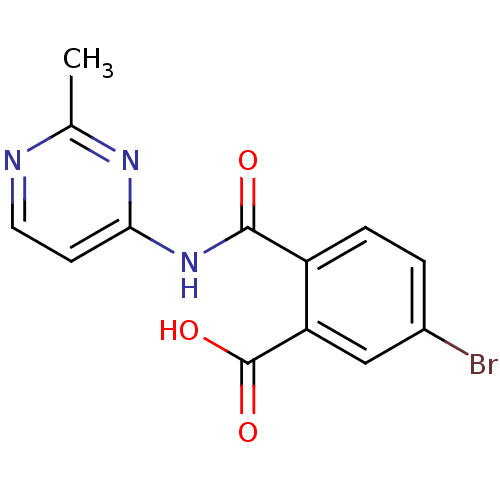 (CHEMBL3098765 | US10195186, Example 36 | US9682967...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | US Patent | 467 | n/a | 490 | n/a | n/a | n/a | n/a | 7.4 | n/a |
H. Lundbeck A/S US Patent | Assay Description The Sortilin assay was performed in total volume of 40 ul in 50 mM HEPES pH 7.4 assay buffer containing 100 mM NaCl, 2.0 mM CaCl2, 0.1% BSA and 0.1% ... | US Patent US9682967 (2017) BindingDB Entry DOI: 10.7270/Q22805SC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurotensin receptor 3 (Homo sapiens (Human)) | BDBM50445044 (CHEMBL3098765 | US10195186, Example 36 | US9682967...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | US Patent | 714 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
H. Lundbeck A/S US Patent | Assay Description The Sortilin assay was performed in total volume of 40 ul in 50 mM HEPES pH 7.4 assay buffer containing 100 mM NaCl, 2.0 mM CaCl2, 0.1% BSA and 0.1% ... | US Patent US10195186 (2019) BindingDB Entry DOI: 10.7270/Q2474CZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurotensin receptor 3 (Homo sapiens (Human)) | BDBM50445044 (CHEMBL3098765 | US10195186, Example 36 | US9682967...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | US Patent | n/a | n/a | 750 | n/a | n/a | n/a | n/a | n/a | n/a |
H. Lundbeck A/S US Patent | Assay Description The Sortilin assay was performed in total volume of 40 ul in 50 mM HEPES pH 7.4 assay buffer containing 100 mM NaCl, 2.0 mM CaCl2, 0.1% BSA and 0.1% ... | US Patent US10195186 (2019) BindingDB Entry DOI: 10.7270/Q2474CZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurotensin receptor 3 (Homo sapiens (Human)) | BDBM50445044 (CHEMBL3098765 | US10195186, Example 36 | US9682967...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 490 | n/a | n/a | n/a | n/a | n/a | n/a |
H. Lundbeck A/S Curated by ChEMBL | Assay Description Displacement of [3H]-neurotensin from sortilin (unknown origin) incubated 30 mins prior to [3H]-neurotensin addition measured after 6 hrs by scintill... | Bioorg Med Chem Lett 24: 177-80 (2013) Article DOI: 10.1016/j.bmcl.2013.11.046 BindingDB Entry DOI: 10.7270/Q2RN39BB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||