 Found 19 hits for monomerid = 50445877
Found 19 hits for monomerid = 50445877 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Nuclear receptor ROR-gamma
(Homo sapiens (Human)) | BDBM50445877
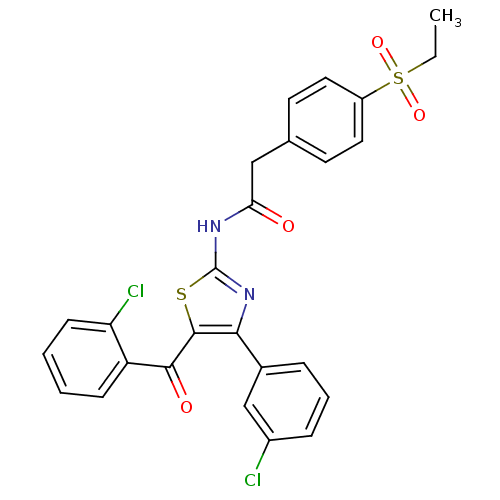
(CHEMBL3105681)Show SMILES CCS(=O)(=O)c1ccc(CC(=O)Nc2nc(c(s2)C(=O)c2ccccc2Cl)-c2cccc(Cl)c2)cc1 Show InChI InChI=1S/C26H20Cl2N2O4S2/c1-2-36(33,34)19-12-10-16(11-13-19)14-22(31)29-26-30-23(17-6-5-7-18(27)15-17)25(35-26)24(32)20-8-3-4-9-21(20)28/h3-13,15H,2,14H2,1H3,(H,29,30,31) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of 25-[26,27-3H]hydroxycholesterol from RORgammat receptor ligand binding domain (unknown origin) after 60 mins |
Bioorg Med Chem 22: 692-702 (2014)
Article DOI: 10.1016/j.bmc.2013.12.021
BindingDB Entry DOI: 10.7270/Q2Z039M7 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Nuclear receptor ROR-gamma
(Homo sapiens (Human)) | BDBM50445877

(CHEMBL3105681)Show SMILES CCS(=O)(=O)c1ccc(CC(=O)Nc2nc(c(s2)C(=O)c2ccccc2Cl)-c2cccc(Cl)c2)cc1 Show InChI InChI=1S/C26H20Cl2N2O4S2/c1-2-36(33,34)19-12-10-16(11-13-19)14-22(31)29-26-30-23(17-6-5-7-18(27)15-17)25(35-26)24(32)20-8-3-4-9-21(20)28/h3-13,15H,2,14H2,1H3,(H,29,30,31) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inverse agonist activity at RORgammat in human TH17 cells assessed as inhibition of IL17 release incubated for 4 days by HTRF assay |
J Med Chem 61: 7796-7813 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00783
BindingDB Entry DOI: 10.7270/Q23R0WFQ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Nuclear receptor ROR-beta
(Homo sapiens (Human)) | BDBM50445877

(CHEMBL3105681)Show SMILES CCS(=O)(=O)c1ccc(CC(=O)Nc2nc(c(s2)C(=O)c2ccccc2Cl)-c2cccc(Cl)c2)cc1 Show InChI InChI=1S/C26H20Cl2N2O4S2/c1-2-36(33,34)19-12-10-16(11-13-19)14-22(31)29-26-30-23(17-6-5-7-18(27)15-17)25(35-26)24(32)20-8-3-4-9-21(20)28/h3-13,15H,2,14H2,1H3,(H,29,30,31) | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 79 | n/a | n/a | n/a | n/a | n/a | n/a |
Phenex Pharmaceuticals AG
Curated by ChEMBL
| Assay Description
Inverse agonist activity at recombinant N-terminally GST-tagged RORbeta ligand binding domain (unknown origin) expressed in Escherichia coli incubate... |
Bioorg Med Chem Lett 24: 5265-7 (2014)
Article DOI: 10.1016/j.bmcl.2014.09.053
BindingDB Entry DOI: 10.7270/Q2S46TKZ |
More data for this
Ligand-Target Pair | |
Nuclear receptor ROR-beta
(Homo sapiens (Human)) | BDBM50445877

(CHEMBL3105681)Show SMILES CCS(=O)(=O)c1ccc(CC(=O)Nc2nc(c(s2)C(=O)c2ccccc2Cl)-c2cccc(Cl)c2)cc1 Show InChI InChI=1S/C26H20Cl2N2O4S2/c1-2-36(33,34)19-12-10-16(11-13-19)14-22(31)29-26-30-23(17-6-5-7-18(27)15-17)25(35-26)24(32)20-8-3-4-9-21(20)28/h3-13,15H,2,14H2,1H3,(H,29,30,31) | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 79.4 | n/a | n/a | n/a | n/a | n/a | n/a |
Phenex Pharmaceuticals AG
Curated by ChEMBL
| Assay Description
Inverse agonist activity at recombinant N-terminally GST-tagged RORbeta ligand binding domain (unknown origin) expressed in Escherichia coli incubate... |
Bioorg Med Chem Lett 24: 5265-7 (2014)
Article DOI: 10.1016/j.bmcl.2014.09.053
BindingDB Entry DOI: 10.7270/Q2S46TKZ |
More data for this
Ligand-Target Pair | |
Nuclear receptor ROR-gamma
(Homo sapiens (Human)) | BDBM50445877

(CHEMBL3105681)Show SMILES CCS(=O)(=O)c1ccc(CC(=O)Nc2nc(c(s2)C(=O)c2ccccc2Cl)-c2cccc(Cl)c2)cc1 Show InChI InChI=1S/C26H20Cl2N2O4S2/c1-2-36(33,34)19-12-10-16(11-13-19)14-22(31)29-26-30-23(17-6-5-7-18(27)15-17)25(35-26)24(32)20-8-3-4-9-21(20)28/h3-13,15H,2,14H2,1H3,(H,29,30,31) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Phenex Pharmaceuticals AG
Curated by ChEMBL
| Assay Description
Inverse agonist activity at RORgammaT (unknown origin) by M1H assay |
Bioorg Med Chem Lett 24: 5265-7 (2014)
Article DOI: 10.1016/j.bmcl.2014.09.053
BindingDB Entry DOI: 10.7270/Q2S46TKZ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Nuclear receptor ROR-alpha
(Homo sapiens (Human)) | BDBM50445877

(CHEMBL3105681)Show SMILES CCS(=O)(=O)c1ccc(CC(=O)Nc2nc(c(s2)C(=O)c2ccccc2Cl)-c2cccc(Cl)c2)cc1 Show InChI InChI=1S/C26H20Cl2N2O4S2/c1-2-36(33,34)19-12-10-16(11-13-19)14-22(31)29-26-30-23(17-6-5-7-18(27)15-17)25(35-26)24(32)20-8-3-4-9-21(20)28/h3-13,15H,2,14H2,1H3,(H,29,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | <5.01E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Phenex Pharmaceuticals AG
Curated by ChEMBL
| Assay Description
Inverse agonist activity at RORalpha (unknown origin) by M1H assay |
Bioorg Med Chem Lett 24: 5265-7 (2014)
Article DOI: 10.1016/j.bmcl.2014.09.053
BindingDB Entry DOI: 10.7270/Q2S46TKZ |
More data for this
Ligand-Target Pair | |
Nuclear receptor ROR-beta
(Homo sapiens (Human)) | BDBM50445877

(CHEMBL3105681)Show SMILES CCS(=O)(=O)c1ccc(CC(=O)Nc2nc(c(s2)C(=O)c2ccccc2Cl)-c2cccc(Cl)c2)cc1 Show InChI InChI=1S/C26H20Cl2N2O4S2/c1-2-36(33,34)19-12-10-16(11-13-19)14-22(31)29-26-30-23(17-6-5-7-18(27)15-17)25(35-26)24(32)20-8-3-4-9-21(20)28/h3-13,15H,2,14H2,1H3,(H,29,30,31) | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 63 | n/a | n/a | n/a | n/a | n/a | n/a |
Phenex Pharmaceuticals AG
Curated by ChEMBL
| Assay Description
Inverse agonist activity at RORbeta (unknown origin) by M1H assay |
Bioorg Med Chem Lett 24: 5265-7 (2014)
Article DOI: 10.1016/j.bmcl.2014.09.053
BindingDB Entry DOI: 10.7270/Q2S46TKZ |
More data for this
Ligand-Target Pair | |
Nuclear receptor subfamily 1 group I member 2
(Homo sapiens (Human)) | BDBM50445877

(CHEMBL3105681)Show SMILES CCS(=O)(=O)c1ccc(CC(=O)Nc2nc(c(s2)C(=O)c2ccccc2Cl)-c2cccc(Cl)c2)cc1 Show InChI InChI=1S/C26H20Cl2N2O4S2/c1-2-36(33,34)19-12-10-16(11-13-19)14-22(31)29-26-30-23(17-6-5-7-18(27)15-17)25(35-26)24(32)20-8-3-4-9-21(20)28/h3-13,15H,2,14H2,1H3,(H,29,30,31) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 260 | n/a | n/a | n/a | n/a |
Phenex Pharmaceuticals AG
Curated by ChEMBL
| Assay Description
Agonist activity at human pregnane X receptor expressed in HEK293 cells by luciferase reporter gene assay |
Bioorg Med Chem Lett 24: 5265-7 (2014)
Article DOI: 10.1016/j.bmcl.2014.09.053
BindingDB Entry DOI: 10.7270/Q2S46TKZ |
More data for this
Ligand-Target Pair | |
Nuclear receptor ROR-gamma
(Homo sapiens (Human)) | BDBM50445877

(CHEMBL3105681)Show SMILES CCS(=O)(=O)c1ccc(CC(=O)Nc2nc(c(s2)C(=O)c2ccccc2Cl)-c2cccc(Cl)c2)cc1 Show InChI InChI=1S/C26H20Cl2N2O4S2/c1-2-36(33,34)19-12-10-16(11-13-19)14-22(31)29-26-30-23(17-6-5-7-18(27)15-17)25(35-26)24(32)20-8-3-4-9-21(20)28/h3-13,15H,2,14H2,1H3,(H,29,30,31) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 8 | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inverse agonist activity at human RoRc-LBD fusion protein with GST expressed in BL-21 (BL3) cells assessed as SRC1 coactivator peptide recruitment |
J Med Chem 57: 5871-92 (2014)
Article DOI: 10.1021/jm401901d
BindingDB Entry DOI: 10.7270/Q2M0473Z |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Nuclear receptor ROR-gamma
(Mus musculus) | BDBM50445877

(CHEMBL3105681)Show SMILES CCS(=O)(=O)c1ccc(CC(=O)Nc2nc(c(s2)C(=O)c2ccccc2Cl)-c2cccc(Cl)c2)cc1 Show InChI InChI=1S/C26H20Cl2N2O4S2/c1-2-36(33,34)19-12-10-16(11-13-19)14-22(31)29-26-30-23(17-6-5-7-18(27)15-17)25(35-26)24(32)20-8-3-4-9-21(20)28/h3-13,15H,2,14H2,1H3,(H,29,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 158 | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Activity at RoRc in mouse CD4+ T cells assessed as inhibition of IL-17 production |
J Med Chem 57: 5871-92 (2014)
Article DOI: 10.1021/jm401901d
BindingDB Entry DOI: 10.7270/Q2M0473Z |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Nuclear receptor ROR-gamma
(Homo sapiens (Human)) | BDBM50445877

(CHEMBL3105681)Show SMILES CCS(=O)(=O)c1ccc(CC(=O)Nc2nc(c(s2)C(=O)c2ccccc2Cl)-c2cccc(Cl)c2)cc1 Show InChI InChI=1S/C26H20Cl2N2O4S2/c1-2-36(33,34)19-12-10-16(11-13-19)14-22(31)29-26-30-23(17-6-5-7-18(27)15-17)25(35-26)24(32)20-8-3-4-9-21(20)28/h3-13,15H,2,14H2,1H3,(H,29,30,31) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 10 | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Activity at human RoRc in human Jurkat cells assessed as inhibition of IL-17 promoter |
J Med Chem 57: 5871-92 (2014)
Article DOI: 10.1021/jm401901d
BindingDB Entry DOI: 10.7270/Q2M0473Z |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Nuclear receptor ROR-gamma
(Mus musculus) | BDBM50445877

(CHEMBL3105681)Show SMILES CCS(=O)(=O)c1ccc(CC(=O)Nc2nc(c(s2)C(=O)c2ccccc2Cl)-c2cccc(Cl)c2)cc1 Show InChI InChI=1S/C26H20Cl2N2O4S2/c1-2-36(33,34)19-12-10-16(11-13-19)14-22(31)29-26-30-23(17-6-5-7-18(27)15-17)25(35-26)24(32)20-8-3-4-9-21(20)28/h3-13,15H,2,14H2,1H3,(H,29,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of RORgammat receptor ligand binding domain in mouse spleen CD4+ T cells assessed as inhibition of IL-17 production after 3 days by ELISA |
Bioorg Med Chem 22: 692-702 (2014)
Article DOI: 10.1016/j.bmc.2013.12.021
BindingDB Entry DOI: 10.7270/Q2Z039M7 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Nuclear receptor ROR-gamma
(Homo sapiens (Human)) | BDBM50445877

(CHEMBL3105681)Show SMILES CCS(=O)(=O)c1ccc(CC(=O)Nc2nc(c(s2)C(=O)c2ccccc2Cl)-c2cccc(Cl)c2)cc1 Show InChI InChI=1S/C26H20Cl2N2O4S2/c1-2-36(33,34)19-12-10-16(11-13-19)14-22(31)29-26-30-23(17-6-5-7-18(27)15-17)25(35-26)24(32)20-8-3-4-9-21(20)28/h3-13,15H,2,14H2,1H3,(H,29,30,31) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of APC-labeled RORgammat receptor ligand binding domain (unknown origin) after 1 hr by FRET assay |
Bioorg Med Chem 22: 692-702 (2014)
Article DOI: 10.1016/j.bmc.2013.12.021
BindingDB Entry DOI: 10.7270/Q2Z039M7 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Retinoic acid receptor gamma
(Homo sapiens (Human)) | BDBM50445877

(CHEMBL3105681)Show SMILES CCS(=O)(=O)c1ccc(CC(=O)Nc2nc(c(s2)C(=O)c2ccccc2Cl)-c2cccc(Cl)c2)cc1 Show InChI InChI=1S/C26H20Cl2N2O4S2/c1-2-36(33,34)19-12-10-16(11-13-19)14-22(31)29-26-30-23(17-6-5-7-18(27)15-17)25(35-26)24(32)20-8-3-4-9-21(20)28/h3-13,15H,2,14H2,1H3,(H,29,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Galderma R&D
Curated by ChEMBL
| Assay Description
Inverse agonist activity at GAL4 DBD-fused RARgamma LBD (unknown origin) expressed in human HG5LN cells after 18 hrs by luciferase reporter gene assa... |
Bioorg Med Chem Lett 26: 5802-5808 (2016)
Article DOI: 10.1016/j.bmcl.2016.10.023
BindingDB Entry DOI: 10.7270/Q2D79DDH |
More data for this
Ligand-Target Pair | |
Nuclear receptor ROR-gamma
(Homo sapiens (Human)) | BDBM50445877

(CHEMBL3105681)Show SMILES CCS(=O)(=O)c1ccc(CC(=O)Nc2nc(c(s2)C(=O)c2ccccc2Cl)-c2cccc(Cl)c2)cc1 Show InChI InChI=1S/C26H20Cl2N2O4S2/c1-2-36(33,34)19-12-10-16(11-13-19)14-22(31)29-26-30-23(17-6-5-7-18(27)15-17)25(35-26)24(32)20-8-3-4-9-21(20)28/h3-13,15H,2,14H2,1H3,(H,29,30,31) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Galderma R&D
Curated by ChEMBL
| Assay Description
Inverse agonist activity at GAL4 DBD-fused RORgammat LBD (unknown origin) expressed in HG5LN cells after 18 hrs by luciferase reporter gene assay |
Bioorg Med Chem Lett 26: 5802-5808 (2016)
Article DOI: 10.1016/j.bmcl.2016.10.023
BindingDB Entry DOI: 10.7270/Q2D79DDH |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Nuclear receptor ROR-gamma
(Homo sapiens (Human)) | BDBM50445877

(CHEMBL3105681)Show SMILES CCS(=O)(=O)c1ccc(CC(=O)Nc2nc(c(s2)C(=O)c2ccccc2Cl)-c2cccc(Cl)c2)cc1 Show InChI InChI=1S/C26H20Cl2N2O4S2/c1-2-36(33,34)19-12-10-16(11-13-19)14-22(31)29-26-30-23(17-6-5-7-18(27)15-17)25(35-26)24(32)20-8-3-4-9-21(20)28/h3-13,15H,2,14H2,1H3,(H,29,30,31) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inverse agonist activity at biotinylated HN-Avi-MBP-TCS-human RORgammat (258 to 518 residues) assessed as inhibition of biotinylated SRC-1 peptide NC... |
J Med Chem 61: 7796-7813 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00783
BindingDB Entry DOI: 10.7270/Q23R0WFQ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Nuclear receptor ROR-gamma
(Homo sapiens (Human)) | BDBM50445877

(CHEMBL3105681)Show SMILES CCS(=O)(=O)c1ccc(CC(=O)Nc2nc(c(s2)C(=O)c2ccccc2Cl)-c2cccc(Cl)c2)cc1 Show InChI InChI=1S/C26H20Cl2N2O4S2/c1-2-36(33,34)19-12-10-16(11-13-19)14-22(31)29-26-30-23(17-6-5-7-18(27)15-17)25(35-26)24(32)20-8-3-4-9-21(20)28/h3-13,15H,2,14H2,1H3,(H,29,30,31) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of [3H]-2-(4-(ethylsulfonyl)phenyl)-N-(4-(2-(methoxymethyl)phenyl)thiophen-2-yl)acetamide from purified N-(HN)6-GST-TCS-human RORgammat ... |
J Med Chem 61: 7796-7813 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00783
BindingDB Entry DOI: 10.7270/Q23R0WFQ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Nuclear receptor ROR-gamma
(Homo sapiens (Human)) | BDBM50445877

(CHEMBL3105681)Show SMILES CCS(=O)(=O)c1ccc(CC(=O)Nc2nc(c(s2)C(=O)c2ccccc2Cl)-c2cccc(Cl)c2)cc1 Show InChI InChI=1S/C26H20Cl2N2O4S2/c1-2-36(33,34)19-12-10-16(11-13-19)14-22(31)29-26-30-23(17-6-5-7-18(27)15-17)25(35-26)24(32)20-8-3-4-9-21(20)28/h3-13,15H,2,14H2,1H3,(H,29,30,31) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Phenex Pharmaceuticals AG
Curated by ChEMBL
| Assay Description
Inverse agonist activity at human recombinant N-terminally 6xHis-tagged RORgamma ligand binding domain (unknown origin) expressed in Escherichia coli... |
Bioorg Med Chem Lett 24: 5265-7 (2014)
Article DOI: 10.1016/j.bmcl.2014.09.053
BindingDB Entry DOI: 10.7270/Q2S46TKZ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Nuclear receptor ROR-gamma
(Homo sapiens (Human)) | BDBM50445877

(CHEMBL3105681)Show SMILES CCS(=O)(=O)c1ccc(CC(=O)Nc2nc(c(s2)C(=O)c2ccccc2Cl)-c2cccc(Cl)c2)cc1 Show InChI InChI=1S/C26H20Cl2N2O4S2/c1-2-36(33,34)19-12-10-16(11-13-19)14-22(31)29-26-30-23(17-6-5-7-18(27)15-17)25(35-26)24(32)20-8-3-4-9-21(20)28/h3-13,15H,2,14H2,1H3,(H,29,30,31) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Phenex Pharmaceuticals AG
Curated by ChEMBL
| Assay Description
Inverse agonist activity at RORgammaT (unknown origin) by Jurkat cell based luciferase assay |
Bioorg Med Chem Lett 24: 5265-7 (2014)
Article DOI: 10.1016/j.bmcl.2014.09.053
BindingDB Entry DOI: 10.7270/Q2S46TKZ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data