

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50446269 CHEMBL3109209
SMILES: Fc1ccc(cc1NC(=O)Nc1cccc(c1)-c1c[nH]c2ncc(cc12)-c1cccnc1)C(F)(F)F
InChI Key: InChIKey=HQPGNAQSHLIGDV-UHFFFAOYSA-N
Data: 3 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Receptor-interacting serine/threonine-protein kinase 1 (Homo sapiens (Human)) | BDBM50446269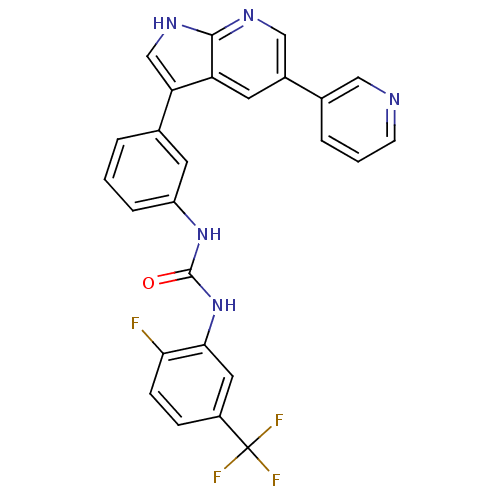 (CHEMBL3109209) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Displacement of (14-(2-{[3-({2-{[4-(cyanomethyl)phenyl]amino}-6-[(5-cyclopropyl-1H-pyrazol-3-yl)amino]-4-pyrimidinyl}amino)propyl]amino}-2-oxoethyl)-... | ACS Med Chem Lett 4: 1238-43 (2013) Article DOI: 10.1021/ml400382p BindingDB Entry DOI: 10.7270/Q2C24XW2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-interacting serine/threonine-protein kinase 1 (Homo sapiens (Human)) | BDBM50446269 (CHEMBL3109209) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human N-terminal His-GST-TEV-fused RIP1 kinase domain (1 to 375) autophosphorylation expressed in baculovirus infected insect Sf9 cells... | ACS Med Chem Lett 4: 1238-43 (2013) Article DOI: 10.1021/ml400382p BindingDB Entry DOI: 10.7270/Q2C24XW2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-interacting serine/threonine-protein kinase 1 (Homo sapiens (Human)) | BDBM50446269 (CHEMBL3109209) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of RIP1 in human U937 cells assessed as prevention of TNFalpha/zVAD.fmk-induced necrotic cell death preincubated for 30 to 60 mins followe... | ACS Med Chem Lett 4: 1238-43 (2013) Article DOI: 10.1021/ml400382p BindingDB Entry DOI: 10.7270/Q2C24XW2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||