

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50447858 CHEBI:75630::CHEMBL501490
SMILES: O[C@H]1Cc2c(O)cc(O)c([C@H]3[C@H](O)[C@H](Oc4cc(O)cc(O)c34)c3ccc(O)c(O)c3)c2O[C@@H]1c1ccc(O)c(O)c1
InChI Key: InChIKey=XFZJEEAOWLFHDH-AVFWISQGSA-N
Data: 1 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| alpha-Glucosidase (α-Glucosidase) (Rattus norvegicus (Rat)) | BDBM50447858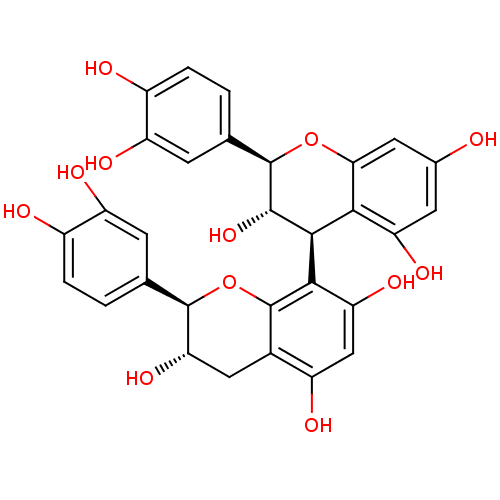 (CHEBI:75630 | CHEMBL501490) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.30E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University Curated by ChEMBL | Assay Description Inhibition of rat intestinal sucrase using p-nitrophenyl-alpha-d-glucopyranoside as substrate incubated for 10 mins prior to substrate addition measu... | Bioorg Med Chem Lett 24: 1192-6 (2014) Article DOI: 10.1016/j.bmcl.2013.12.098 BindingDB Entry DOI: 10.7270/Q2TM7CMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||