

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50448745 CHEMBL3128013::US8835445, 26
SMILES: Cc1cc2nc(-c3nccs3)n(-c3cc4nc(N)nc(N)c4cc3C)c2cc1C
InChI Key: InChIKey=ZSJCDAXDBJWWDN-UHFFFAOYSA-N
Data: 4 KI
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dihydrofolate Reductase (DHFR) (Staphylococcus aureus) | BDBM50448745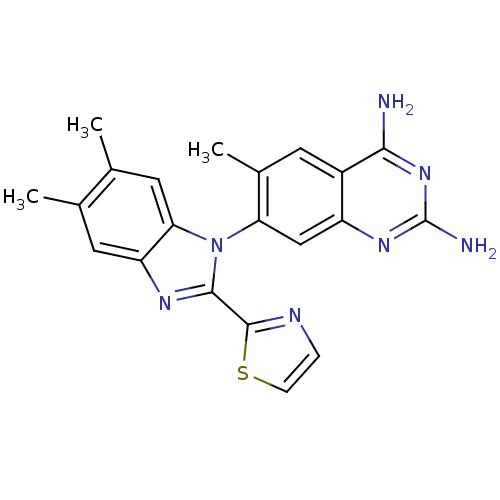 (CHEMBL3128013 | US8835445, 26) | PDB MMDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trius Therapeutics, Inc. US Patent | Assay Description Antibacterial activity as measured by the minimal inhibitory concentrations (MIC) and minimal bactericidal concentrations of compounds are well known... | US Patent US8835445 (2014) BindingDB Entry DOI: 10.7270/Q2DB80J0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate Reductase (DHFR) (Staphylococcus aureus) | BDBM50448745 (CHEMBL3128013 | US8835445, 26) | PDB MMDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trius Therapeutics Inc. Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus DHFR using dihydrofolate as substrate preincubated for 10 mins followed by substrate addition by spectrophotometr... | J Med Chem 57: 651-68 (2014) Article DOI: 10.1021/jm401204g BindingDB Entry DOI: 10.7270/Q2PN974G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Homo sapiens (Human)) | BDBM50448745 (CHEMBL3128013 | US8835445, 26) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trius Therapeutics Inc. Curated by ChEMBL | Assay Description Inhibition of human DHFR using dihydrofolate as substrate preincubated for 10 mins followed by substrate addition by spectrophotometric analysis in p... | J Med Chem 57: 651-68 (2014) Article DOI: 10.1021/jm401204g BindingDB Entry DOI: 10.7270/Q2PN974G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Homo sapiens (Human)) | BDBM50448745 (CHEMBL3128013 | US8835445, 26) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trius Therapeutics, Inc. US Patent | Assay Description Antibacterial activity as measured by the minimal inhibitory concentrations (MIC) and minimal bactericidal concentrations of compounds are well known... | US Patent US8835445 (2014) BindingDB Entry DOI: 10.7270/Q2DB80J0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||