

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50448969 CHEMBL3125517::US9296736, 256::US9593129, Example 256
SMILES: CN(C[C@H](C1CC1)N1[C@@H]([C@H](C[C@](C)(CC(O)=O)C1=O)c1cccc(Cl)c1)c1ccc(Cl)cc1)S(=O)(=O)C1CC1
InChI Key: InChIKey=GNBHDBPUJCJXQG-GCMXZSHTSA-N
Data: 6 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MDM2 and p53 (Homo sapiens (Human)) | BDBM50448969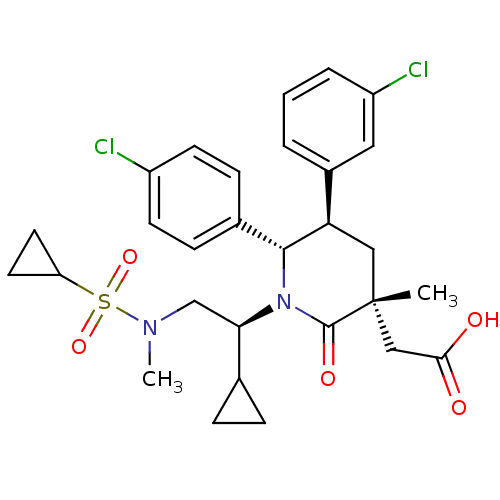 (CHEMBL3125517 | US9296736, 256 | US9593129, Exampl...) | PDB MMDB KEGG B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Amgen INC. US Patent | Assay Description The standard assay conditions for the in vitro HTRF assay consisted of a 50 ul total reaction volume in black 384-well Costar polypropylene plates in... | US Patent US9296736 (2016) BindingDB Entry DOI: 10.7270/Q29022M2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| MDM2 and p53 (Homo sapiens (Human)) | BDBM50448969 (CHEMBL3125517 | US9296736, 256 | US9593129, Exampl...) | PDB MMDB KEGG B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Amgen INC. US Patent | Assay Description The standard assay conditions for the in vitro HTRF assay consisted of a 50 ul total reaction volume in black 384-well Costar polypropylene plates in... | US Patent US9296736 (2016) BindingDB Entry DOI: 10.7270/Q29022M2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| MDM2-MDMX (Homo sapiens (Human)) | BDBM50448969 (CHEMBL3125517 | US9296736, 256 | US9593129, Exampl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
International Institute for Translational Chinese Medicine Curated by ChEMBL | Assay Description Inhibition of p53-MDM2 interaction (unknown origin) by HTRF assay | Eur J Med Chem 159: 1-9 (2018) Article DOI: 10.1016/j.ejmech.2018.09.044 BindingDB Entry DOI: 10.7270/Q2SB48FV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (aa 1-188) (Homo sapiens (Human)) | BDBM50448969 (CHEMBL3125517 | US9296736, 256 | US9593129, Exampl...) | PDB GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. US Patent | Assay Description The standard assay conditions for the in vitro HTRF assay consisted of a 50 ul total reaction volume in black 384-well Costar polypropylene plates in... | US Patent US9593129 (2017) BindingDB Entry DOI: 10.7270/Q20P1232 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (aa 1-188) (Homo sapiens (Human)) | BDBM50448969 (CHEMBL3125517 | US9296736, 256 | US9593129, Exampl...) | PDB GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. US Patent | Assay Description The standard assay conditions for the in vitro HTRF assay consisted of a 50 ul total reaction volume in black 384-well Costar polypropylene plates in... | US Patent US9593129 (2017) BindingDB Entry DOI: 10.7270/Q20P1232 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM50448969 (CHEMBL3125517 | US9296736, 256 | US9593129, Exampl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Binding affinity to human GST-thrombin-tagged MDM2 assessed as inhibition of interaction with human p53 after 1 hr by HTRF assay | J Med Chem 57: 1454-72 (2014) Article DOI: 10.1021/jm401753e BindingDB Entry DOI: 10.7270/Q24M960Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||