

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM52237 2-methyl-7-[phenyl-(2-pyridinylamino)methyl]-8-quinolinol::2-methyl-7-[phenyl-(2-pyridylamino)methyl]quinolin-8-ol::2-methyl-7-[phenyl-(pyridin-2-ylamino)methyl]quinolin-8-ol::MLS000522853::Oxyquinoline, D2, #5::SMR000128119::cid_4999481
SMILES: Cc1ccc2ccc(C(Nc3ccccn3)c3ccccc3)c(O)c2n1
InChI Key: InChIKey=JXFRSLRXSBGCHN-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| streptokinase A precursor (Streptococcus pyogenes M1 GAS) | BDBM52237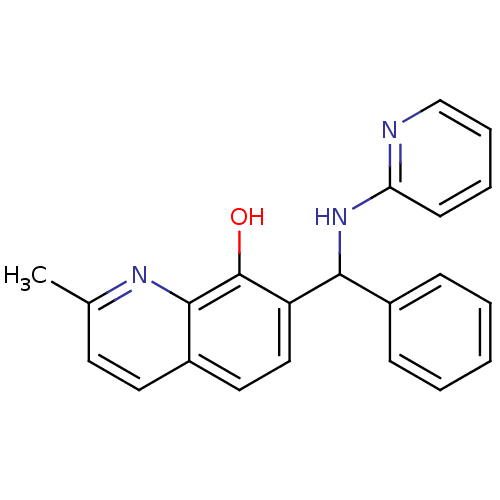 (2-methyl-7-[phenyl-(2-pyridinylamino)methyl]-8-qui...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | PCBioAssay | n/a | n/a | n/a | n/a | 1.50E+5 | n/a | n/a | n/a | n/a |
Broad Institute Curated by PubChem BioAssay | Assay Description Keywords: Group A streptococcus, GAS, streptokinase, expression, virulence, inhibition, dose response, EC50 Assay Overview: The goal of this assa... | PubChem Bioassay (2009) BindingDB Entry DOI: 10.7270/Q2736PBV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM52237 (2-methyl-7-[phenyl-(2-pyridinylamino)methyl]-8-qui...) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
U.S. Army Medical Research Institute of Infectious Diseases Curated by ChEMBL | Assay Description Inhibition of protease activity of recombinant full length Clostridium botulinum Hall BoNT/A light chain using SNAP-25 peptide (187 to 203 residues) ... | Bioorg Med Chem Lett 27: 675-678 (2017) BindingDB Entry DOI: 10.7270/Q2NP26PM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hypoxia-inducible factor 1-alpha (Homo sapiens (Human)) | BDBM52237 (2-methyl-7-[phenyl-(2-pyridinylamino)methyl]-8-qui...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Weill Medical College of Cornell University | Assay Description The predication of no specific interaction between enzyme and iron-binding inhibitors but the Enzyme inhibition constants (IC50s)will change in paral... | Chem Biol 17: 380-91 (2010) Article DOI: 10.1016/j.chembiol.2010.03.008 BindingDB Entry DOI: 10.7270/Q20K271K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||