

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM5292 2-({4-[(4-methanesulfonylpiperidin-1-yl)methyl]pyridin-2-yl}amino)-1,3-thiazole-5-carbonitrile::2-[(4-{[4-(Methylsulfonyl)piperidin-1-yl]methyl}pyridin-2-yl)amino]-1,3-thiazole-5-carbonitrile::N-(1,3-Thiazol-2-yl)pyridin-2-amine 22
SMILES: CS(=O)(=O)C1CCN(Cc2ccnc(Nc3ncc(s3)C#N)c2)CC1
InChI Key: InChIKey=MAUCYALFIUOTRM-UHFFFAOYSA-N
Data: 1 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM5292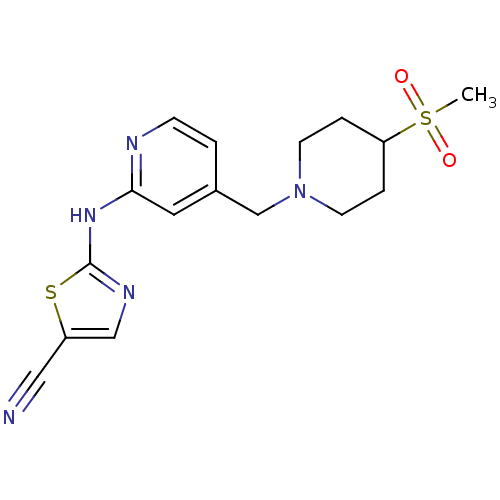 (2-({4-[(4-methanesulfonylpiperidin-1-yl)methyl]pyr...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Merck Research Laboratories | Assay Description Activated KDR was incubated with 25 uM/10 uCi of [gamma-33P] ATP, poly-Glu/Tyr, and inhibitors in kinase buffer for 15 min at 22 °C. The reactio... | J Med Chem 47: 6363-72 (2004) Article DOI: 10.1021/jm049697f BindingDB Entry DOI: 10.7270/Q2PG1PXD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||