

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM54140 MLS001043248::N-[(5Z)-4-keto-5-(2-ketoindolin-3-ylidene)-2-thioxo-thiazolidin-3-yl]benzamide::N-[(5Z)-4-oxidanylidene-5-(2-oxidanylidene-1H-indol-3-ylidene)-2-sulfanylidene-1,3-thiazolidin-3-yl]benzamide::N-[(5Z)-4-oxo-5-(2-oxo-1H-indol-3-ylidene)-2-sulfanylidene-1,3-thiazolidin-3-yl]benzamide::N-[(5Z)-4-oxo-5-(2-oxo-1H-indol-3-ylidene)-2-sulfanylidene-3-thiazolidinyl]benzamide::N-{4-Oxo-5-[2-oxo-1,2-dihydro-indol-(3Z)-ylidene]-2-thioxo-thiazolidin-3-yl}-benzamide::SMR000425314::cid_5457058
SMILES: O=C(NN1C(=S)S\C(C1=O)=C1/C(=O)Nc2ccccc12)c1ccccc1
InChI Key: InChIKey=SRIKEVHBJPZBFZ-YPKPFQOOSA-N
Data: 6 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Zinc aminopeptidase (Plasmodium falciparum (isolate FcB1 / Columbia)) | BDBM54140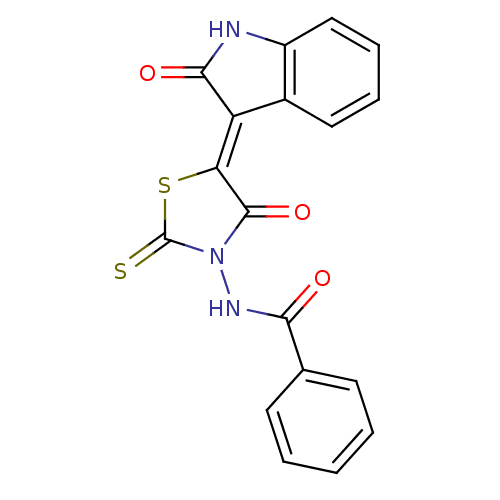 (MLS001043248 | N-[(5Z)-4-keto-5-(2-ketoindolin-3-y...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | PCBioAssay | n/a | n/a | 1.12E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
SRMLSC Curated by PubChem BioAssay | Assay Description Southern Research Molecular Libraries Screening Center (SRMLSC) Southern Research Institute (Birmingham, Alabama) NIH Molecular Libraries Screening... | PubChem Bioassay (2008) BindingDB Entry DOI: 10.7270/Q27H1H0J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM54140 (MLS001043248 | N-[(5Z)-4-keto-5-(2-ketoindolin-3-y...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | PCBioAssay | n/a | n/a | 3.83E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Molecular Screening Center Curated by PubChem BioAssay | Assay Description Source (MLPCN Center Name): The Scripps Research Institute Molecular Screening Center Affiliation: The Scripps Research Institute, TSRI Assay Provide... | PubChem Bioassay (2009) BindingDB Entry DOI: 10.7270/Q2NZ8631 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpi (Rattus norvegicus (Rat)) | BDBM54140 (MLS001043248 | N-[(5Z)-4-keto-5-(2-ketoindolin-3-y...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | PCBioAssay | n/a | n/a | 6.46E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Burnham Center for Chemical Genomics Curated by PubChem BioAssay | Assay Description Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute (SBMRI, San Diego, C... | PubChem Bioassay (2010) BindingDB Entry DOI: 10.7270/Q2DB809V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alkaline phosphatase placental-like (Homo sapiens (Human)) | BDBM54140 (MLS001043248 | N-[(5Z)-4-keto-5-(2-ketoindolin-3-y...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | PCBioAssay | n/a | n/a | 5.31E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Burnham Center for Chemical Genomics Curated by PubChem BioAssay | Assay Description Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute (SBMRI, San Diego, C... | PubChem Bioassay (2010) BindingDB Entry DOI: 10.7270/Q2NS0SCW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alkaline phosphatase, tissue-nonspecific isozyme (Homo sapiens (Human)) | BDBM54140 (MLS001043248 | N-[(5Z)-4-keto-5-(2-ketoindolin-3-y...) | KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | PCBioAssay | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Burnham Center for Chemical Genomics Curated by PubChem BioAssay | Assay Description Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute (SBMRI, San Diego, C... | PubChem Bioassay (2010) BindingDB Entry DOI: 10.7270/Q2H130G1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Intestinal alkaline phosphatase (Homo sapiens (Human)) | BDBM54140 (MLS001043248 | N-[(5Z)-4-keto-5-(2-ketoindolin-3-y...) | PDB MMDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | PCBioAssay | n/a | n/a | 3.09E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Burnham Center for Chemical Genomics Curated by PubChem BioAssay | Assay Description Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute(SBMRI, San Diego, CA... | PubChem Bioassay (2010) BindingDB Entry DOI: 10.7270/Q2X63KDM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||