

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM5443 6-Thiazolylquinazoline 5::N-[4-(benzyloxy)-3-chlorophenyl]-6-(2-{[(2-methanesulfonylethyl)amino]methyl}-1,3-thiazol-4-yl)quinazolin-4-amine
SMILES: CS(=O)(=O)CCNCc1nc(cs1)-c1ccc2ncnc(Nc3ccc(OCc4ccccc4)c(Cl)c3)c2c1
InChI Key: InChIKey=AXVKGZLOECWENV-UHFFFAOYSA-N
Data: 2 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Receptor tyrosine-protein kinase erbB-2 (Homo sapiens (Human)) | BDBM5443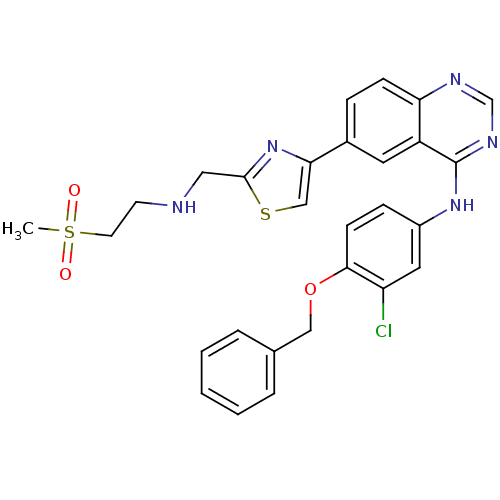 (6-Thiazolylquinazoline 5 | N-[4-(benzyloxy)-3-chlo...) | PDB MMDB UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | 7.5 | 23 |
GlaxoSmithKline | Assay Description Enzymatic reactions were initiated by adding kinase to the reaction mixture containing ATP, [gamma-33P] ATP, peptide substrate and test inhibitor com... | Bioorg Med Chem Lett 14: 111-4 (2004) Article DOI: 10.1016/j.bmcl.2003.10.010 BindingDB Entry DOI: 10.7270/Q2HQ3X4M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM5443 (6-Thiazolylquinazoline 5 | N-[4-(benzyloxy)-3-chlo...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | 7.5 | 23 |
GlaxoSmithKline | Assay Description Enzymatic reactions were initiated by adding kinase to the reaction mixture containing ATP, [gamma-33P] ATP, peptide substrate and test inhibitor com... | Bioorg Med Chem Lett 14: 111-4 (2004) Article DOI: 10.1016/j.bmcl.2003.10.010 BindingDB Entry DOI: 10.7270/Q2HQ3X4M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||