

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM545 4-cyano-N-(3-{1-[4-hydroxy-2-oxo-6-(2-phenylethyl)-6-propyl-5,6-dihydro-2H-pyran-3-yl]propyl}phenyl)benzene-1-sulfonamide::CHEMBL21281::Sulfonamide-Containing 5,6-Dihydro-4-hydroxy-2-pyrones::Tipranavir analog 2
SMILES: CCCC1(CCc2ccccc2)CC(=O)C(C(CC)c2cccc(NS(=O)(=O)c3ccc(cc3)C#N)c2)C(=O)O1
InChI Key: InChIKey=BQKCUXNGMWFRMA-UHFFFAOYSA-N
Data: 3 KI
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HIV-1 Protease (Human immunodeficiency virus type 1) | BDBM545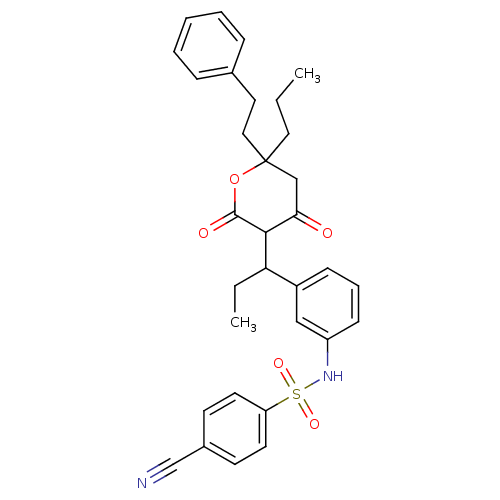 (4-cyano-N-(3-{1-[4-hydroxy-2-oxo-6-(2-phenylethyl)...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8 | -10.9 | n/a | n/a | n/a | n/a | n/a | 5.0 | 22 |
Upjohn | Assay Description HIV-1 protease was purified and refolded from E. coli inclusion bodies. The substrate used spans the p17-p24 processing site (R-V-S-Q-N-Y-P-I-V-Q-N-K... | J Med Chem 39: 4349-53 (1996) Article DOI: 10.1021/jm960541s BindingDB Entry DOI: 10.7270/Q2HQ3X35 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Human immunodeficiency virus type 1 protease (Human immunodeficiency virus type 1) | BDBM545 (4-cyano-N-(3-{1-[4-hydroxy-2-oxo-6-(2-phenylethyl)...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia & Upjohn Curated by ChEMBL | Assay Description Compound was evaluated for in vitro inhibition of human immunodeficiency virus type 1 (HIV-1) Protease | Bioorg Med Chem Lett 8: 1237-42 (1999) BindingDB Entry DOI: 10.7270/Q2K64H7T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| HIV-1 Protease (Human immunodeficiency virus type 1) | BDBM545 (4-cyano-N-(3-{1-[4-hydroxy-2-oxo-6-(2-phenylethyl)...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8.20 | -10.9 | n/a | n/a | n/a | n/a | n/a | 5.0 | 22 |
Upjohn | Assay Description HIV-1 protease was purified and refolded from E. coli inclusion bodies. The substrate used spans the p17-p24 processing site (R-V-S-Q-N-Y-P-I-V-Q-N-K... | J Med Chem 41: 3467-76 (1998) Article DOI: 10.1021/jm9802158 BindingDB Entry DOI: 10.7270/Q2S180P3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||