

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM59099 Bi-ligand, 2
SMILES: Oc1ccc(\C=C2/SC(=S)N(CCCCC(=O)NCc3ccc(Cl)c(Cl)c3)C2=O)cc1O
InChI Key: InChIKey=QJYBFPPSYZFEAR-ODLFYWEKSA-N
Data: 3 KI
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| D-lactate dehydrogenase (Escherichia coli) | BDBM59099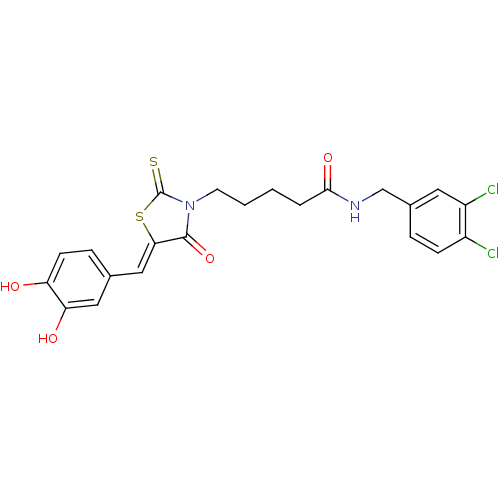 (Bi-ligand, 2) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 42 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Triad Therapeutics, Inc | Assay Description All reactions were monitored spectrophotometrically at 340 nm by using initial rates from the first 5% of reaction. | Chem Biol 11: 185-94 (2004) Article DOI: 10.1016/j.chembiol.2004.02.012 BindingDB Entry DOI: 10.7270/Q2K9360M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 1-deoxy-D-xylulose 5-phosphate reductoisomerase (Escherichia coli) | BDBM59099 (Bi-ligand, 2) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Triad Therapeutics, Inc | Assay Description All reactions were monitored spectrophotometrically at 340 nm by using initial rates from the first 5% of reaction. | Chem Biol 11: 185-94 (2004) Article DOI: 10.1016/j.chembiol.2004.02.012 BindingDB Entry DOI: 10.7270/Q2K9360M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrodipicolinate reductase (DHPR) (Escherichia coli) | BDBM59099 (Bi-ligand, 2) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Triad Therapeutics, Inc | Assay Description All reactions were monitored spectrophotometrically at 340 nm by using initial rates from the first 5% of reaction. | Chem Biol 11: 185-94 (2004) Article DOI: 10.1016/j.chembiol.2004.02.012 BindingDB Entry DOI: 10.7270/Q2K9360M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||