

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM60989 (3S)-2-[(2S,3S)-2-(hydroxymethyl)-3-(3-methoxy-4-oxidanyl-phenyl)-2,3-dihydro-1,4-benzodioxin-6-yl]-3,5,7-tris(oxidanyl)-2,3-dihydrochromen-4-one::(3S)-3,5,7-trihydroxy-2-[(2S,3S)-3-(4-hydroxy-3-methoxy-phenyl)-2-methylol-2,3-dihydro-1,4-benzodioxin-6-yl]chroman-4-one::(3S)-3,5,7-trihydroxy-2-[(2S,3S)-3-(4-hydroxy-3-methoxyphenyl)-2-(hydroxymethyl)-2,3-dihydro-1,4-benzodioxin-6-yl]-2,3-dihydrochromen-4-one::(3S)-3,5,7-trihydroxy-2-[(2S,3S)-3-(4-hydroxy-3-methoxyphenyl)-2-(hydroxymethyl)-2,3-dihydro-1,4-benzodioxin-6-yl]-3,4-dihydro-2H-1-benzopyran-4-one::MLS001332411::SMR000857154::Silibinin::cid_16211710
SMILES: COc1cc(ccc1O)[C@@H]1Oc2cc(ccc2O[C@H]1CO)C1Oc2cc(O)cc(O)c2C(O)C1=O
InChI Key: InChIKey=DAEUJFZPBBHELE-AQXLJOHRSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Carbonic anhydrase 15 (Mus musculus) | BDBM60989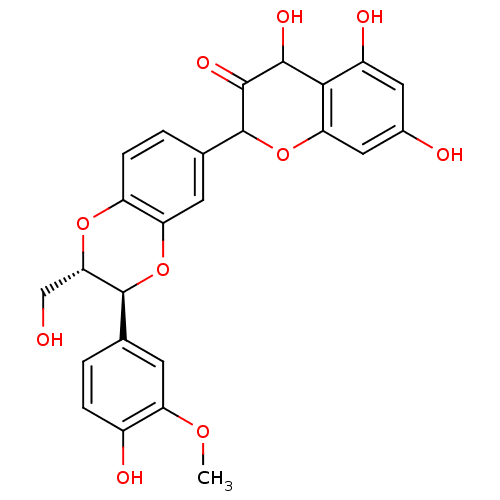 ((3S)-2-[(2S,3S)-2-(hydroxymethyl)-3-(3-methoxy-4-o...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 650 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibition of mouse carbonic anhydrase 15 after 15 mins by stopped flow CO2 hydration method | Bioorg Med Chem Lett 20: 5050-3 (2010) Article DOI: 10.1016/j.bmcl.2010.07.038 BindingDB Entry DOI: 10.7270/Q2HM59FX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM60989 ((3S)-2-[(2S,3S)-2-(hydroxymethyl)-3-(3-methoxy-4-o...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina at Greensboro Curated by ChEMBL | Assay Description Inhibition of CYP2C9 in pooled human liver microsomes assessed as reduction in enzyme-mediated (S)-warfarin 7-hydroxylation by HPLC/MS-MS method | Bioorg Med Chem 21: 742-7 (2013) Article DOI: 10.1016/j.bmc.2012.11.035 BindingDB Entry DOI: 10.7270/Q23R0V65 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Xanthine dehydrogenase/oxidase (Rattus norvegicus) | BDBM60989 ((3S)-2-[(2S,3S)-2-(hydroxymethyl)-3-(3-methoxy-4-o...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.03E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacy School of Wenzhou Medical College Curated by ChEMBL | Assay Description Inhibition of rat liver xanthine oxidase after 2 hrs by spectrophotometry | J Med Chem 52: 7732-52 (2009) Article DOI: 10.1021/jm900735p BindingDB Entry DOI: 10.7270/Q2CF9R2X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||