

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM635 (2R,4S)-2-[(R)-(benzylcarbamoyl)(1-phenylacetamido)methyl]-N-(3-{[(2R,4S)-2-[(R)-(benzylcarbamoyl)(1-phenylacetamido)methyl]-5,5-dimethyl-1,3-thiazolidin-4-yl]formamido}propyl)-5,5-dimethyl-1,3-thiazolidine-4-carboxamide::CHEMBL318844::Penicillin Et(NH)2 Sym dimer ::penicillin deriv. 48
SMILES: [H][C@]1(N[C@@H](C(=O)NCCCNC(=O)[C@@H]2N[C@]([H])(SC2(C)C)[C@H](NC(=O)Cc2ccccc2)C(=O)NCc2ccccc2)C(C)(C)S1)[C@H](NC(=O)Cc1ccccc1)C(=O)NCc1ccccc1
InChI Key: InChIKey=XMIJDZJXXSLMTK-WATWFJIKSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HIV-1 Protease (Human immunodeficiency virus type 1) | BDBM635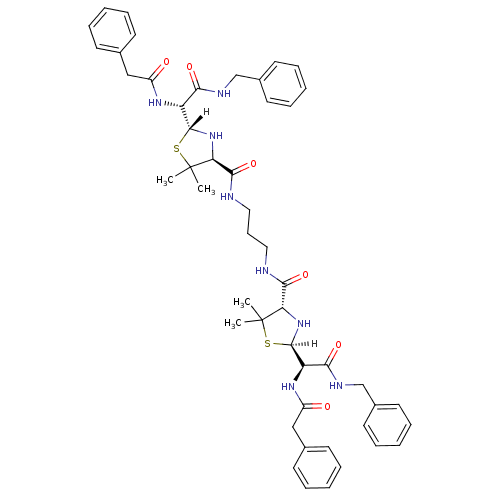 ((2R,4S)-2-[(R)-(benzylcarbamoyl)(1-phenylacetamido...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 540 | n/a | n/a | n/a | n/a | 6.0 | 37 |
Glaxo Group Research Ltd. | Assay Description IC50 values were obtained by assaying the enzyme against the synthetic substrate peptide KQGTVSFNFPQIT, which was tritiated at the proline residue, a... | J Med Chem 36: 3129-36 (1993) Article DOI: 10.1021/jm00073a012 BindingDB Entry DOI: 10.7270/Q20P0X6C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Human immunodeficiency virus type 1 protease (Human immunodeficiency virus type 1) | BDBM635 ((2R,4S)-2-[(R)-(benzylcarbamoyl)(1-phenylacetamido...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 540 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description The compound was tested in vitro for its inhibitory activity against HIV-1 Proteinase activity | Bioorg Med Chem Lett 3: 503-508 (1993) Article DOI: 10.1016/S0960-894X(01)81216-6 BindingDB Entry DOI: 10.7270/Q2GH9HXV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Human immunodeficiency virus type 1 protease (Human immunodeficiency virus type 1) | BDBM635 ((2R,4S)-2-[(R)-(benzylcarbamoyl)(1-phenylacetamido...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 540 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Limited Curated by ChEMBL | Assay Description In vitro inhibitory activity against HIV proteinase | J Med Chem 37: 3707-16 (1994) BindingDB Entry DOI: 10.7270/Q2V40T88 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||