 Found 11 hits for monomerid = 6497
Found 11 hits for monomerid = 6497 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM6497
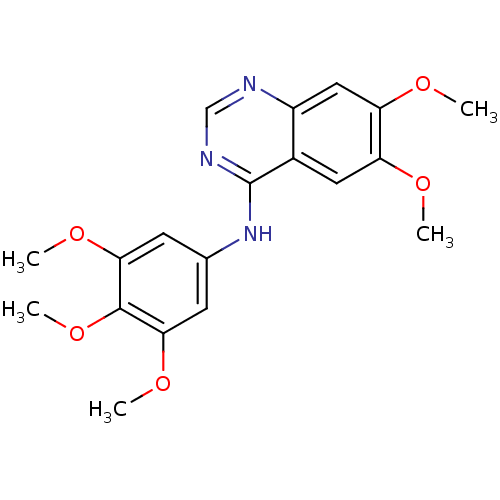
(6,7-dimethoxy-N-(3,4,5-trimethoxyphenyl)quinazolin...)Show InChI InChI=1S/C19H21N3O5/c1-23-14-8-12-13(9-15(14)24-2)20-10-21-19(12)22-11-6-16(25-3)18(27-5)17(7-11)26-4/h6-10H,1-5H3,(H,20,21,22) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 280 | n/a | n/a | n/a | n/a | 7.5 | 30 |
Wyeth-Ayerst Research
| Assay Description
Src kinase activity was measured in an ELISA format. IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the tra... |
Bioorg Med Chem Lett 10: 2477-80 (2000)
Article DOI: 10.1016/S0960-894X(00)00493-5
BindingDB Entry DOI: 10.7270/Q20P0X7T |
More data for this
Ligand-Target Pair | |
Macrophage colony stimulating factor receptor
(Homo sapiens (Human)) | BDBM6497

(6,7-dimethoxy-N-(3,4,5-trimethoxyphenyl)quinazolin...)Show InChI InChI=1S/C19H21N3O5/c1-23-14-8-12-13(9-15(14)24-2)20-10-21-19(12)22-11-6-16(25-3)18(27-5)17(7-11)26-4/h6-10H,1-5H3,(H,20,21,22) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Antagonism towards Colony stimulating factor 1 receptor (CSF-1R) |
Bioorg Med Chem Lett 7: 421-424 (1997)
Article DOI: 10.1016/S0960-894X(97)00035-8
BindingDB Entry DOI: 10.7270/Q2SB45RN |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM6497

(6,7-dimethoxy-N-(3,4,5-trimethoxyphenyl)quinazolin...)Show InChI InChI=1S/C19H21N3O5/c1-23-14-8-12-13(9-15(14)24-2)20-10-21-19(12)22-11-6-16(25-3)18(27-5)17(7-11)26-4/h6-10H,1-5H3,(H,20,21,22) | PDB
UniProtKB/SwissProt
UniProtKB/TrEMBL
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of p56 lck tyrosine kinase |
Bioorg Med Chem Lett 7: 421-424 (1997)
Article DOI: 10.1016/S0960-894X(97)00035-8
BindingDB Entry DOI: 10.7270/Q2SB45RN |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM6497

(6,7-dimethoxy-N-(3,4,5-trimethoxyphenyl)quinazolin...)Show InChI InChI=1S/C19H21N3O5/c1-23-14-8-12-13(9-15(14)24-2)20-10-21-19(12)22-11-6-16(25-3)18(27-5)17(7-11)26-4/h6-10H,1-5H3,(H,20,21,22) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Antagonism towards Epidermal growth factor receptor |
Bioorg Med Chem Lett 7: 421-424 (1997)
Article DOI: 10.1016/S0960-894X(97)00035-8
BindingDB Entry DOI: 10.7270/Q2SB45RN |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM6497

(6,7-dimethoxy-N-(3,4,5-trimethoxyphenyl)quinazolin...)Show InChI InChI=1S/C19H21N3O5/c1-23-14-8-12-13(9-15(14)24-2)20-10-21-19(12)22-11-6-16(25-3)18(27-5)17(7-11)26-4/h6-10H,1-5H3,(H,20,21,22) | PDB
UniProtKB/SwissProt
UniProtKB/TrEMBL
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of p56lck kinase autophosphorylation in Jurkat cells |
Bioorg Med Chem Lett 7: 417-420 (1997)
Article DOI: 10.1016/S0960-894X(97)00034-6
BindingDB Entry DOI: 10.7270/Q2J966VN |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase GAK
(Homo sapiens (Human)) | BDBM6497

(6,7-dimethoxy-N-(3,4,5-trimethoxyphenyl)quinazolin...)Show InChI InChI=1S/C19H21N3O5/c1-23-14-8-12-13(9-15(14)24-2)20-10-21-19(12)22-11-6-16(25-3)18(27-5)17(7-11)26-4/h6-10H,1-5H3,(H,20,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 350 | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina at Chapel Hill
Curated by ChEMBL
| Assay Description
Inhibition of tracer 5 binding to human N-terminal nano luciferase-fused GAK expressed in HEK293 cells measured after 2 hrs by nanoBRET assay |
J Med Chem 62: 4772-4778 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00350 |
More data for this
Ligand-Target Pair | |
PDGFR-beta/Platelet-derived growth factor receptor alpha
(Homo sapiens (Human)) | BDBM6497

(6,7-dimethoxy-N-(3,4,5-trimethoxyphenyl)quinazolin...)Show InChI InChI=1S/C19H21N3O5/c1-23-14-8-12-13(9-15(14)24-2)20-10-21-19(12)22-11-6-16(25-3)18(27-5)17(7-11)26-4/h6-10H,1-5H3,(H,20,21,22) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of platelet-derived growth factor receptor (PDGF-R) |
Bioorg Med Chem Lett 7: 421-424 (1997)
Article DOI: 10.1016/S0960-894X(97)00035-8
BindingDB Entry DOI: 10.7270/Q2SB45RN |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase GAK
(Homo sapiens (Human)) | BDBM6497

(6,7-dimethoxy-N-(3,4,5-trimethoxyphenyl)quinazolin...)Show InChI InChI=1S/C19H21N3O5/c1-23-14-8-12-13(9-15(14)24-2)20-10-21-19(12)22-11-6-16(25-3)18(27-5)17(7-11)26-4/h6-10H,1-5H3,(H,20,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a |
University of North Carolina at Chapel Hill
Curated by ChEMBL
| Assay Description
Binding affinity to wild-type human partial length GAK (G13 to Y338 residues) expressed in bacterial expression system by Kinomescan method |
J Med Chem 62: 4772-4778 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00350 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM6497

(6,7-dimethoxy-N-(3,4,5-trimethoxyphenyl)quinazolin...)Show InChI InChI=1S/C19H21N3O5/c1-23-14-8-12-13(9-15(14)24-2)20-10-21-19(12)22-11-6-16(25-3)18(27-5)17(7-11)26-4/h6-10H,1-5H3,(H,20,21,22) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a |
University of North Carolina at Chapel Hill
Curated by ChEMBL
| Assay Description
Binding affinity to wild-type human partial length EGFR (R669 to V1011 residues) expressed in bacterial expression system by Kinomescan method |
J Med Chem 62: 4772-4778 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00350 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM6497

(6,7-dimethoxy-N-(3,4,5-trimethoxyphenyl)quinazolin...)Show InChI InChI=1S/C19H21N3O5/c1-23-14-8-12-13(9-15(14)24-2)20-10-21-19(12)22-11-6-16(25-3)18(27-5)17(7-11)26-4/h6-10H,1-5H3,(H,20,21,22) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina at Chapel Hill
Curated by ChEMBL
| Assay Description
Inhibition of EGFR in human A431 cells assessed as reduction in EGF-stimulated EGFR autophosphorylation preincuabted for 90 mins followed by EGF-stim... |
J Med Chem 62: 4772-4778 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00350 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM6497

(6,7-dimethoxy-N-(3,4,5-trimethoxyphenyl)quinazolin...)Show InChI InChI=1S/C19H21N3O5/c1-23-14-8-12-13(9-15(14)24-2)20-10-21-19(12)22-11-6-16(25-3)18(27-5)17(7-11)26-4/h6-10H,1-5H3,(H,20,21,22) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Epidermal growth factor receptor autophosphorylation. |
Bioorg Med Chem Lett 7: 417-420 (1997)
Article DOI: 10.1016/S0960-894X(97)00034-6
BindingDB Entry DOI: 10.7270/Q2J966VN |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data